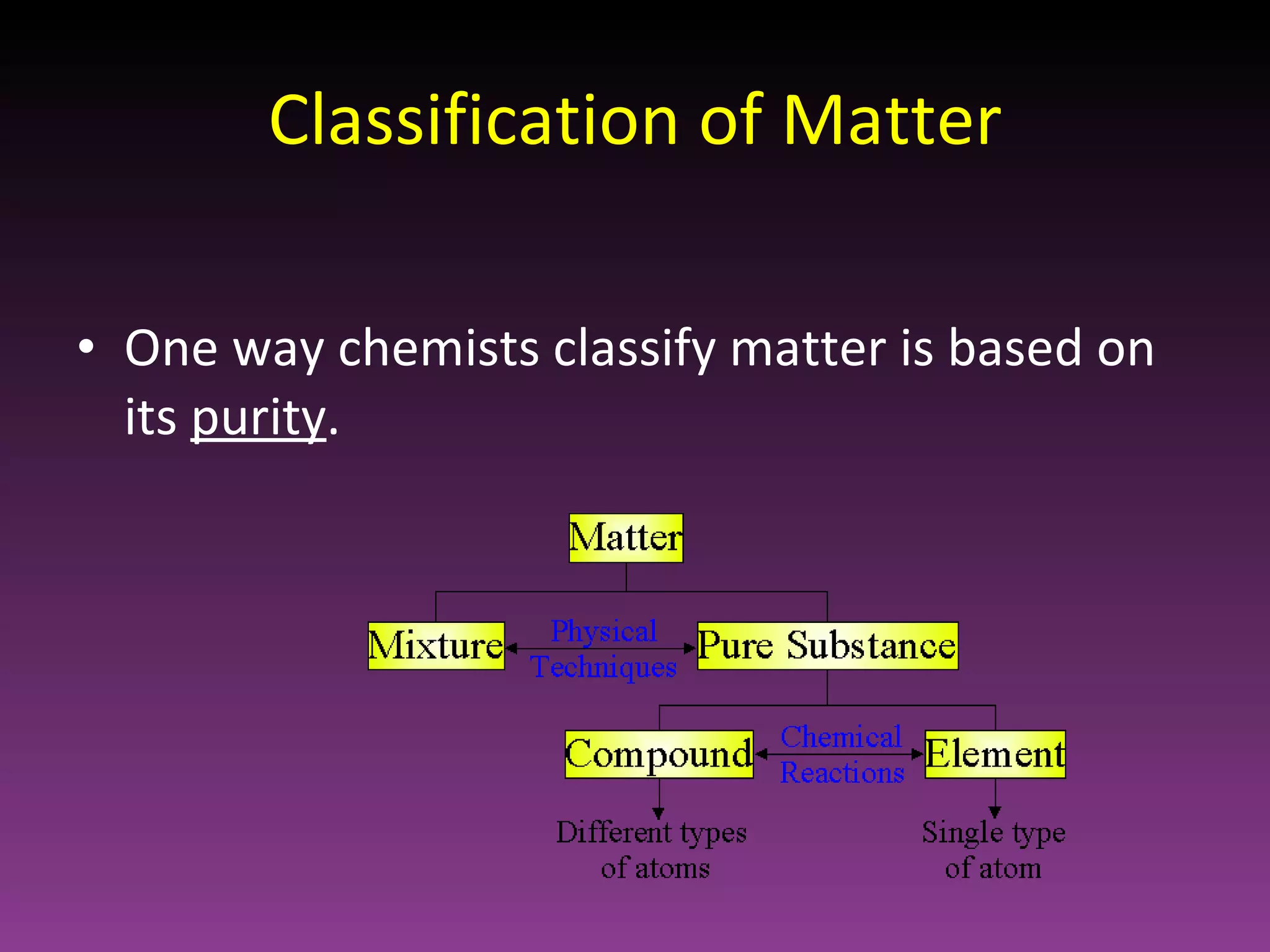
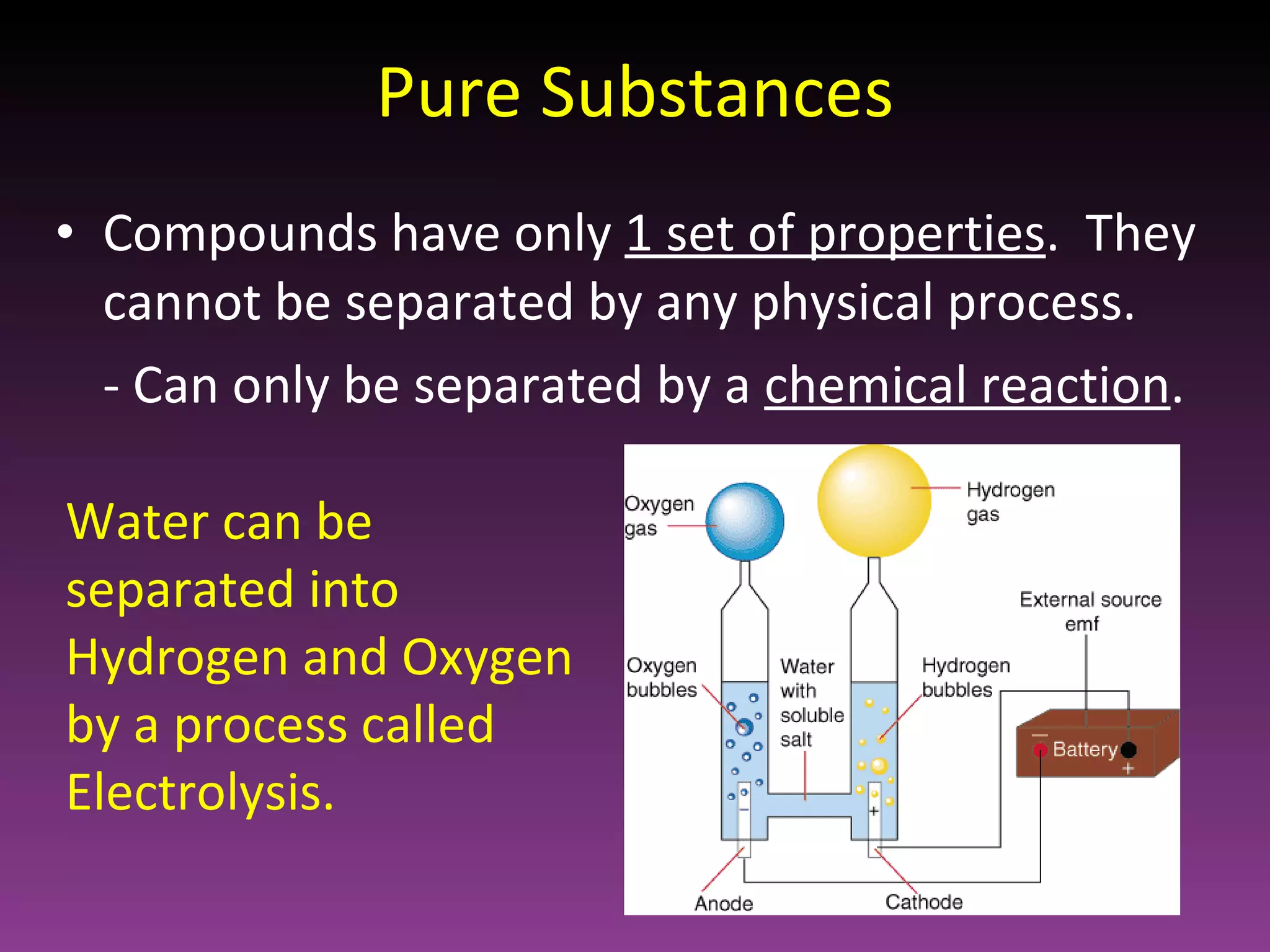
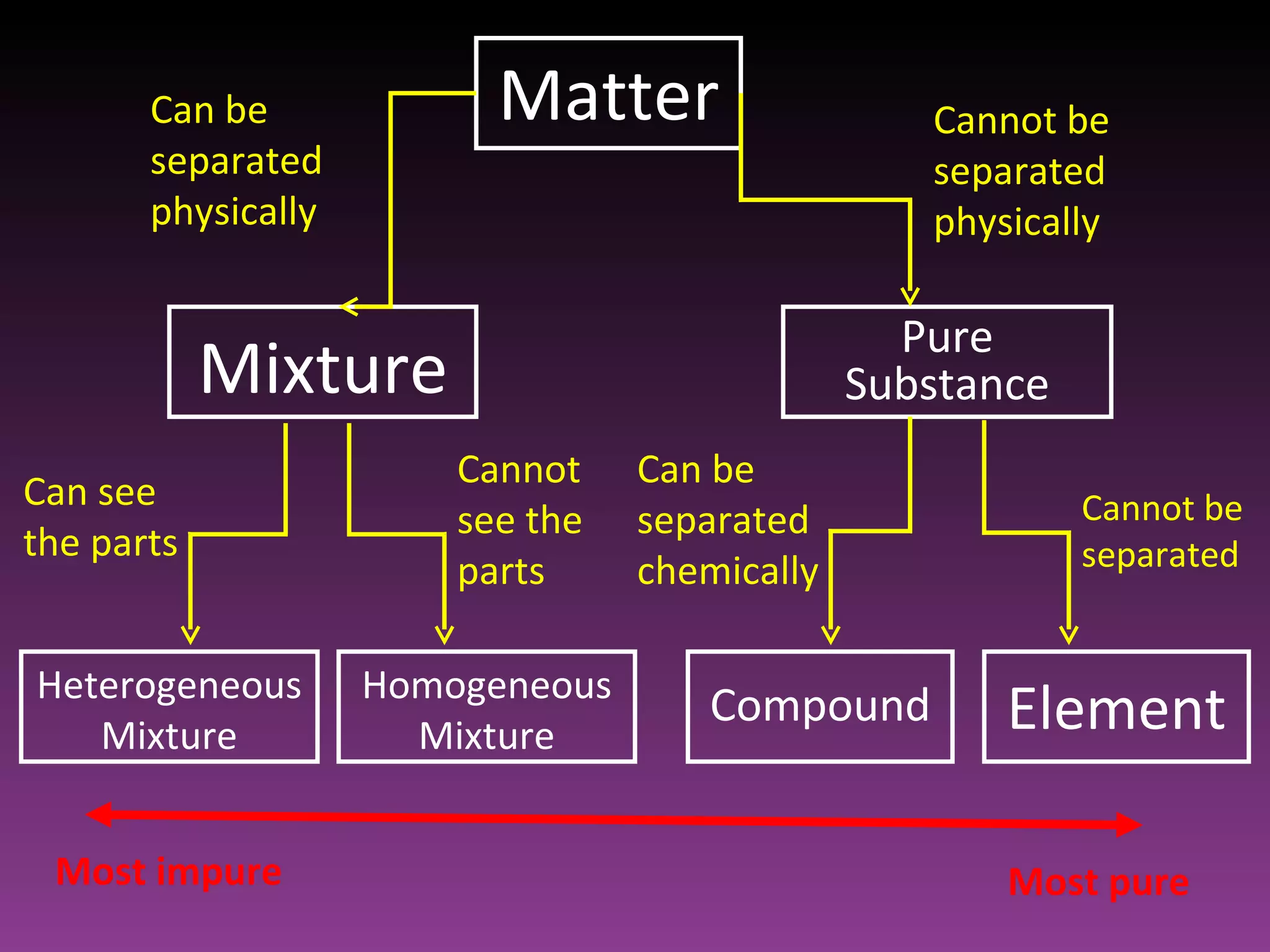
This document classifies matter based on purity and composition. It discusses pure substances like elements and compounds that have a definite composition and properties that cannot be altered through physical means. Mixtures are less pure and have a variable composition, consisting of two or more pure substances that are either homogenously mixed at the atomic level or heterogeneously mixed in an uneven distribution where the parts can be seen.