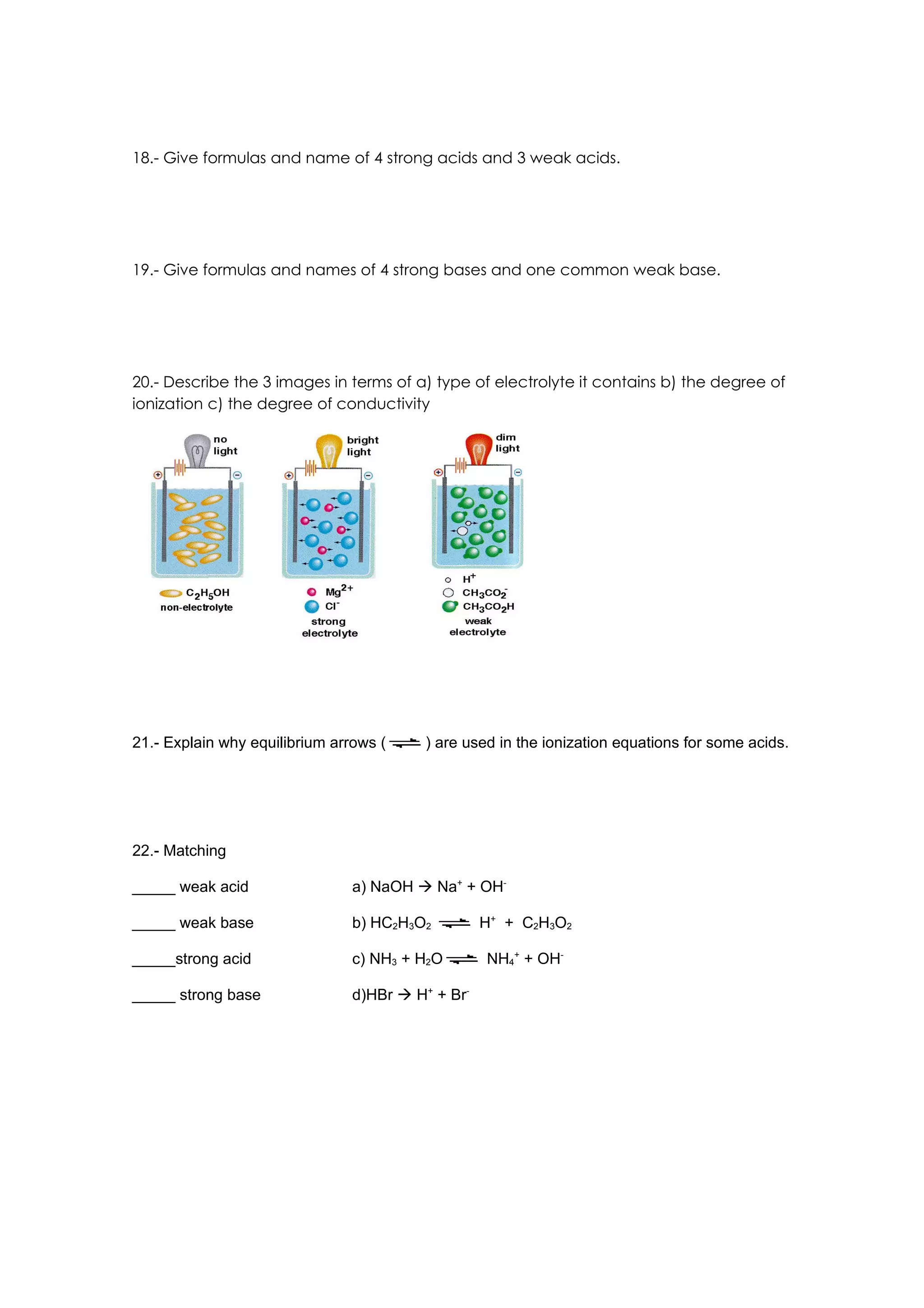
The document contains a review of concepts related to acids and bases including:
1) Acids have a sour taste and react with metals and carbonates to produce gases. They release H+ ions in water and turn litmus paper red.
2) Bases taste bitter and feel slippery. They conduct electricity in solution, react with fats to produce soap, and turn litmus paper blue.
3) The document asks questions about strong vs weak acids/bases, gives examples of each, and discusses ionization in solutions and chemical equations involving acids and bases.