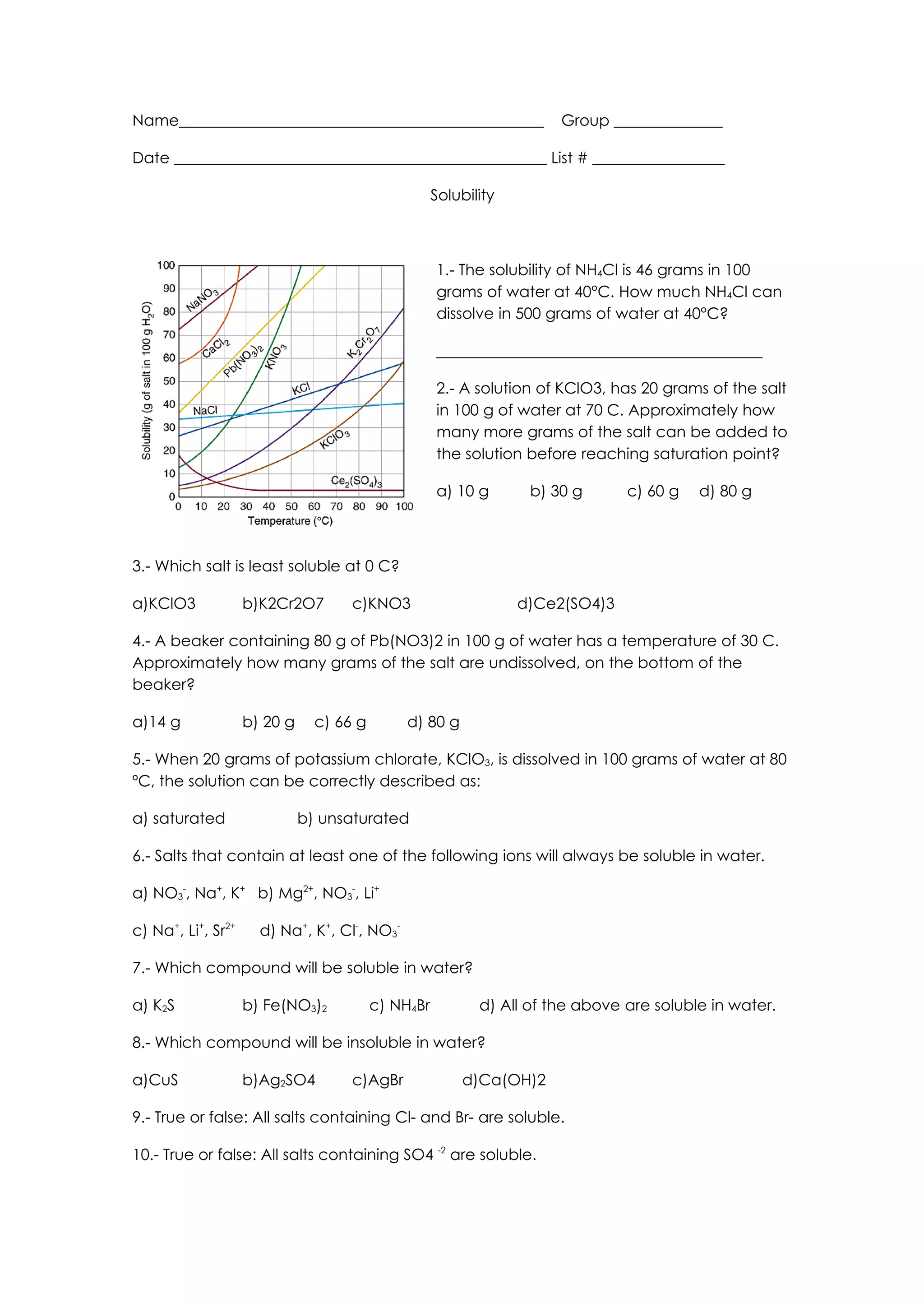
This document contains a chemistry worksheet with 10 multiple choice questions about solubility of salts. The questions cover topics such as calculating how much of a salt can dissolve in a given amount of water, determining saturation points, identifying the least soluble salt at a given temperature, and identifying which ions or compounds result in soluble or insoluble salts.