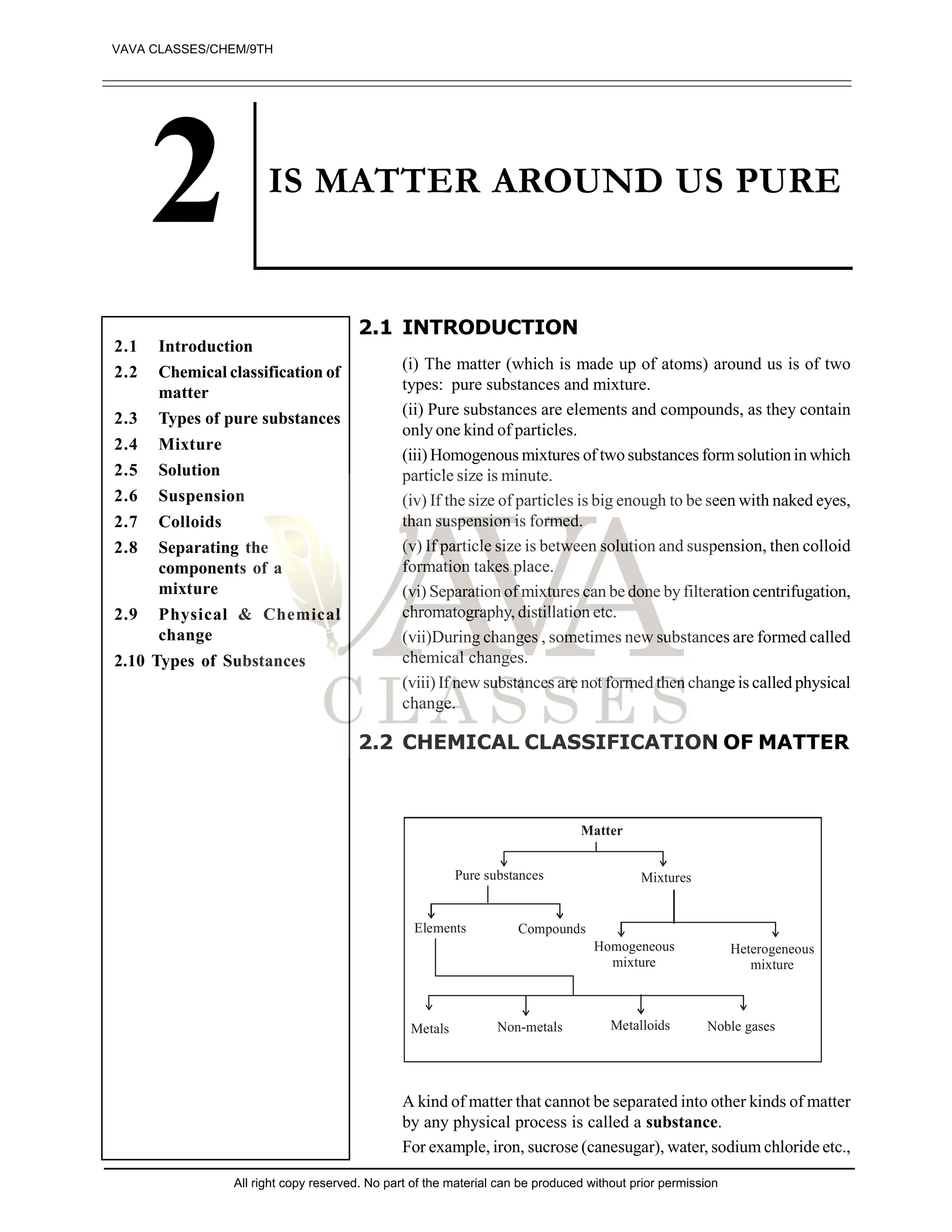
The document discusses the classification of matter into pure substances and mixtures, explaining that pure substances can be elements or compounds, while mixtures can be homogeneous or heterogeneous. It outlines the properties and characteristics of metals, non-metals, and metalloids, alongside definitions and examples of solutions, suspensions, and colloids. The document also emphasizes methods of separation for mixtures and distinguishes between physical and chemical changes.






![EXERCISE-I
1. Is colloidal solution homogeneous in nature? [1]
2. What do we name the zig-zag motion of particles in a colloidal solution? [1]
3. Suggest two ways to separate a mixture of iron filings and sulphur. [1]
4. Which of the following will shows Tyndall effect. [1]
(i) Sodium chloride solution
(ii) Starch solution
(iii) Brass
5. What happens when electric current is passed through a colloidal solution? [1]
6. Does freezing of ice represent a physical change? [1]
7. Compare the particle sizes in true solution, colloidal solution and suspensions. [1]
8. What will happen if the beam of light is passed through a colloidal solution? [1]
9. Is water free from suspended impurities a pure substance? [1]
10. Give one example of a non-aqueous solution.
11. Name the metal which is liquid at room temperature. [1]
12. A substance can be beatan into sheets and drawn into wires. What will you call it? [1]
13. Can a single substance act as a mixture. [1]
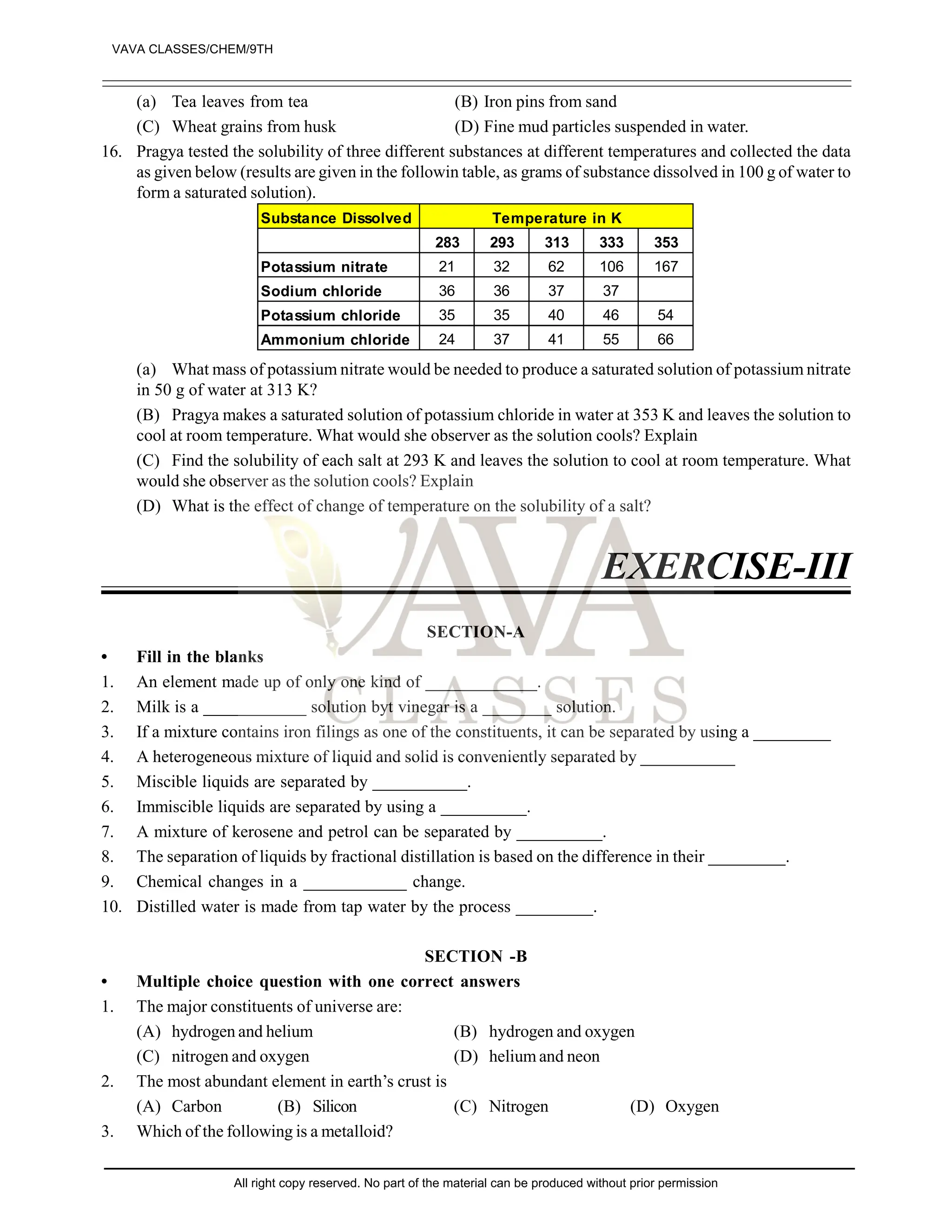
14. 5 g of sodium chloride is dissolved in 100 g of water to form a solution. Is this solution saturated in nature?
[2]
15. 4 g of a solute is dissolved in 36 g of water. What is the mass percent of the solution? [2]
16. What is solubility of a salt? Discuss the effect of temperature on the solubility of sodium chloride in water?
[2]
17. Name the elements which has the characateristics of both metals and non-metals, what we call them?
[2]
18. A combination of common salt and iron filings is a mixture and not a compound. Justify? [2]
19. A mixture contains sand and naphthalene. Both are solids. Suggest a method for their separation [2]
20. A compound is regarded as a pure substance while the mixture not. Assign reason. [2]
21. Give main points of distinction in true solution, colloidal solution and suspension. [3]
22. Bleeding from a fresh cut can be stopped by applying alum. Explain. [3]
23. What do you mean by physical and chemical changes? How will you distinguish them? [3]
24. A mixture of alcohol and water is called a true solution while that of mustard oil and water is known as
emulsion. how will you account for it? [3]
25. Asolution contains 40 g of a salt in 320 g of water. Calculate the concentration in terms of mass percentage
of the solution. [3]
26. To make a saturated solution, 36 g of solution chloride is dissolved in 100 g of water at 293 K. Find its
concentration at this temperature. [3]
27. How will you separate a mixture of common salt, sulphur powder and sand? [5]
28. Describe the steps involved in the separation of iodine, iron filings and salt from a mixture. [5]
29. (a) 9.72 g of potassium chloride dissolved in 30 g of water at 70°C. Calculate the solubility of potassium
chloride at that temperature.
(B) What do you understand by the statement “the solubility of copper sulphate in water at 20°C is 20.7 g”
VAVA CLASSES/CHEM/9TH
All right copy reserved. No part of the material can be produced without prior permission](https://image.slidesharecdn.com/02ismatterarounduspure-240208123118-edacfa6a/75/Class-9-Science-chapter-is-matter-around-us-pure-notes-pdf-21-2048.jpg)
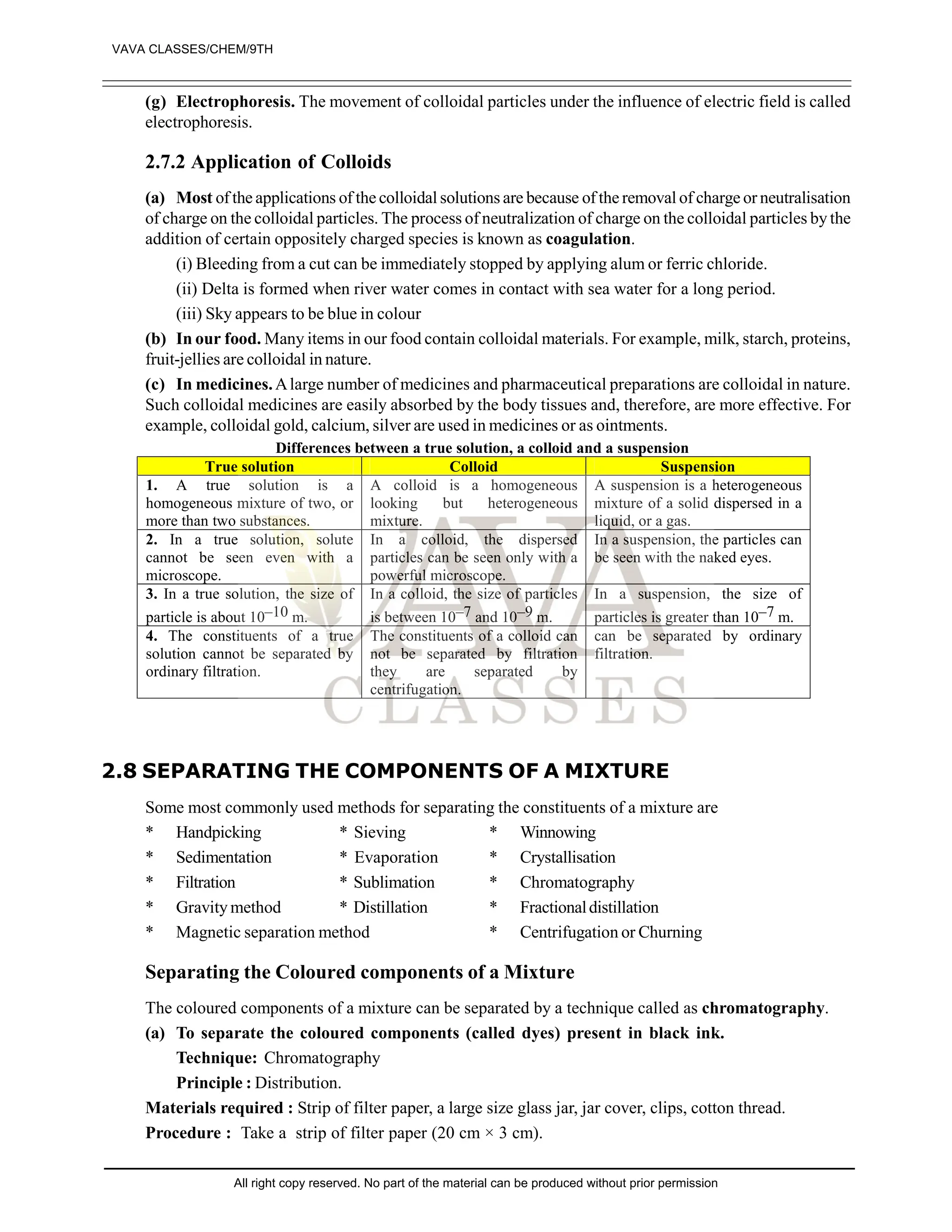
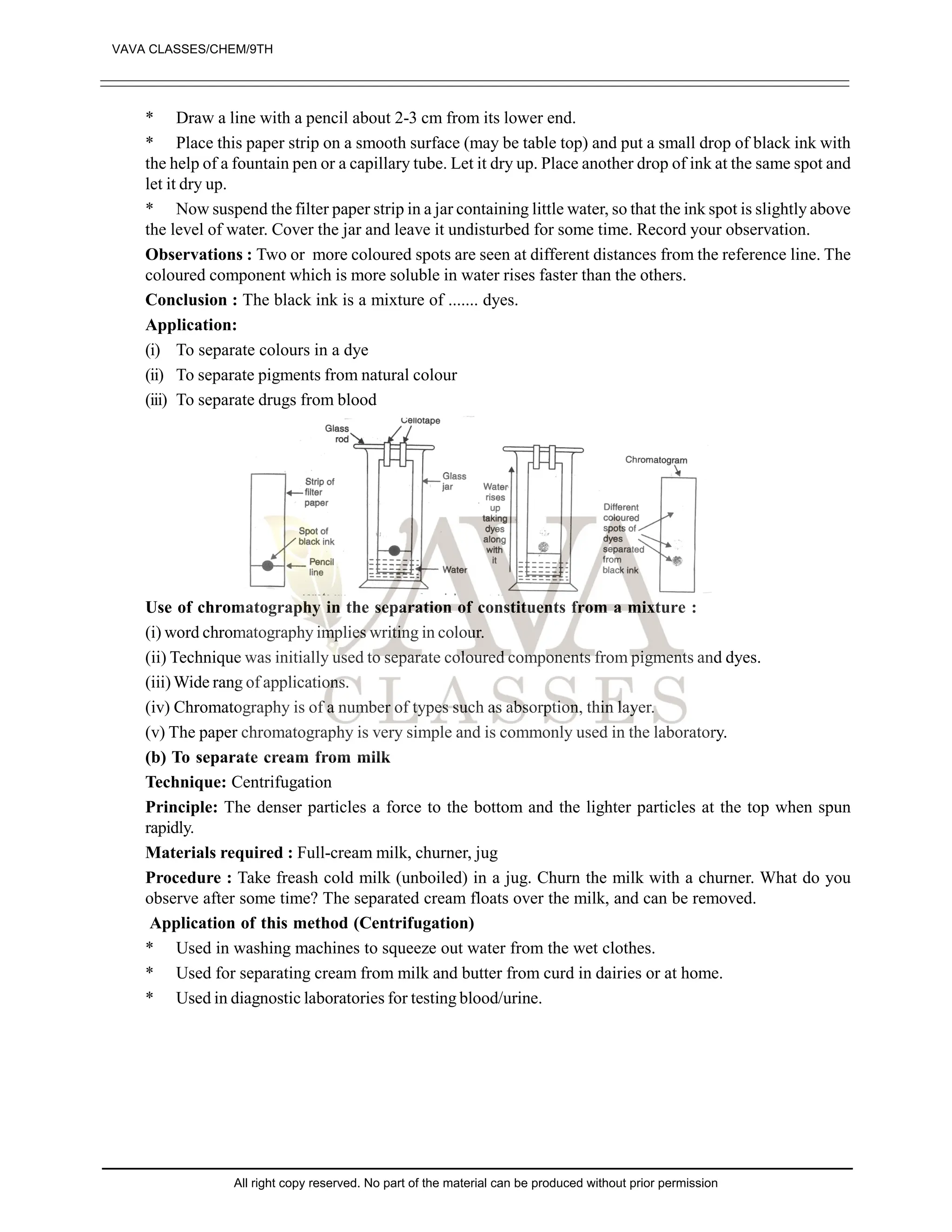
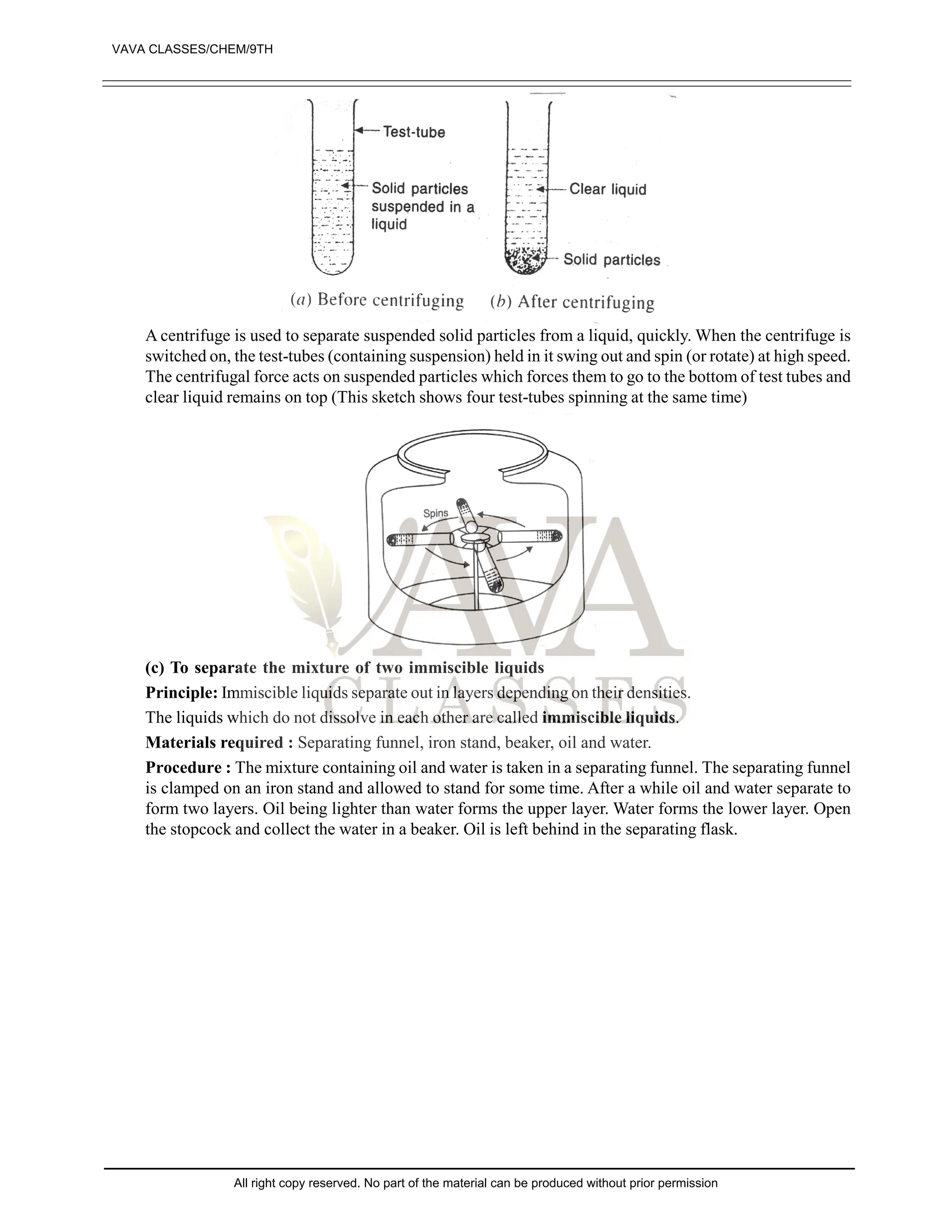
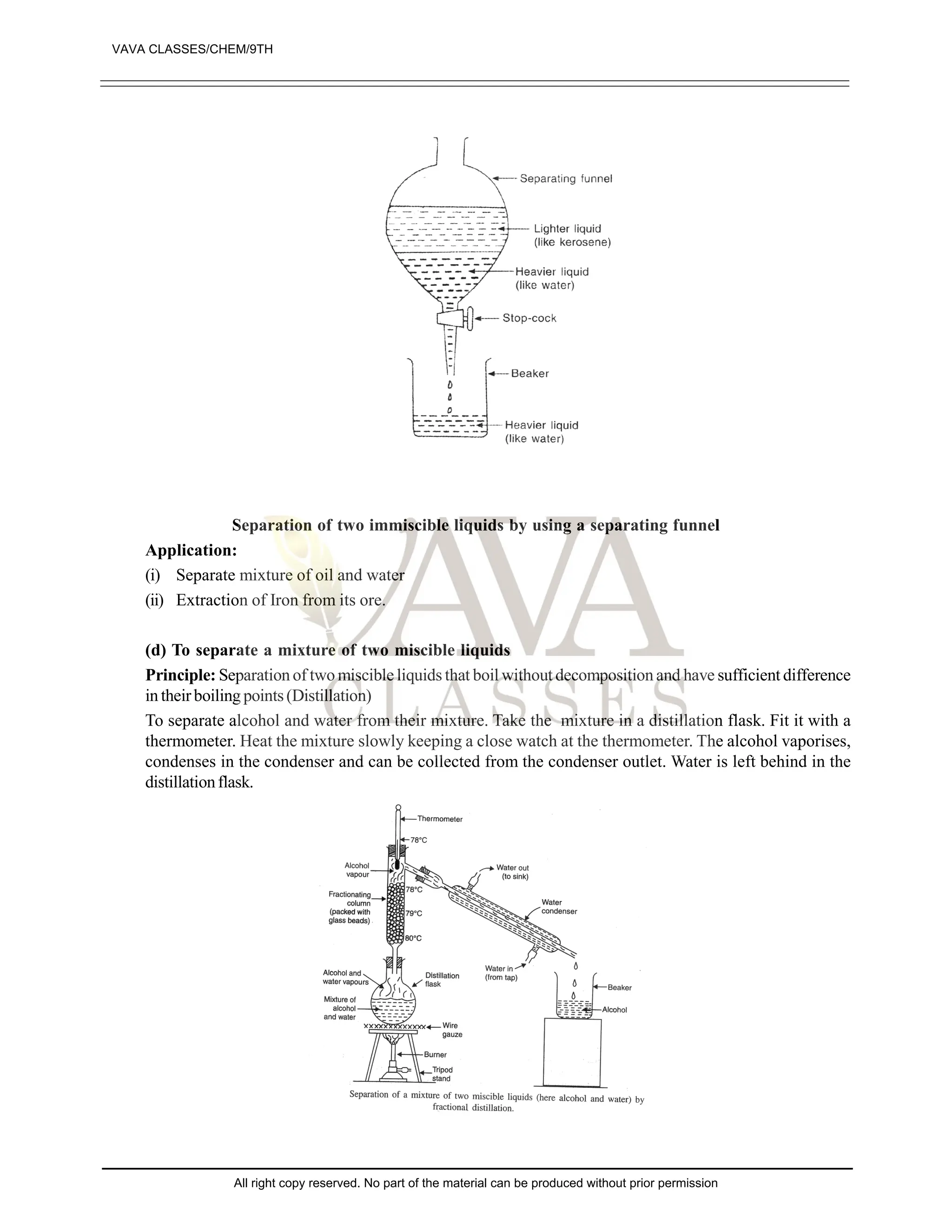
![EXERCISE-II
1. What are pure substances? Give two examples of pure substances. [1]
2. What is a mixture? Give two examples of mixtures. [1]
3. State whether the following statements is true or false: [1]
“Sugar is a pure substance”
4. What is an element? Give examples of two elements. [1]
5. Define a solute and a solvent. [2]
6. Which of the following will show Tyndall and effect, why? [2]
(a) Saltsolution (B) Milk
(C) Copper sulphate (D) Starch solution
7. Classify each of the following as homogenous or heterogenous mixture?
Soda water, wood, air, soil, vinegar, filtered tea.
8. How would you confirm that colourless liquid given to you is pure water? [3]
9. Which of the following materials fall in the category of a “pure substance” and why? [4]
(a) Ice (B) Milk
(C) Iron (D) Hydrochloric acid
(e) Calciumoxide (f) Mercury
(g) Brick (h) Wood
(i) Air
10. Which of the following are chemical changes, why? [4]
(a) Growth of a plant (B) Rusting of iron
(C) Mixing of iron fillings and sand (D) Looking of food
(e) Digestion of food (f) Freezing of water
(g) Burning of candle
11. Classify the following into elements, compounds and mixtures. [4]
(a) Sodium (B) Soil
(C) Sugar solution (D) Silver
(e) Calcium carbonate (f) Tin
(g) Silicon (h) Coal
(i) Air (j) Soap
(k) Methane (l) Carbon dioxide
12. Which separation techniques will you apply for the separation of the following [5]
(a) Sodium chloride from its solution in water
(B) Ammonium chloride from a mixture containing sodium chloride and ammonium chloride
(C) Small pieces of metals in the engine oil of a car
(D) Different pigments from an extract of flower petals
(e) Butter from curd
13. Write the steps you would use for making tea use the word solution, solvent, solute, dissolve soluble,
insoluble, filterate and residue. [5]
14. Explain the process involved in the separation of oil from water, with diagram [5]
15. Make the diagram involved in the separation of following mixtures (labell the diagram) [5]
VAVA CLASSES/CHEM/9TH
All right copy reserved. No part of the material can be produced without prior permission](https://image.slidesharecdn.com/02ismatterarounduspure-240208123118-edacfa6a/75/Class-9-Science-chapter-is-matter-around-us-pure-notes-pdf-22-2048.jpg)