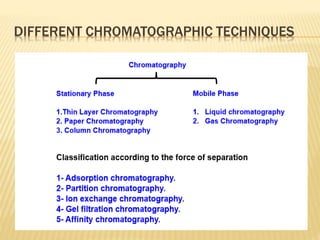
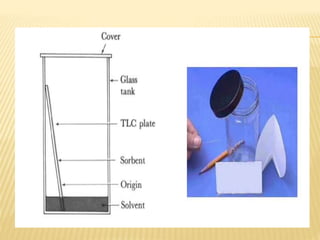
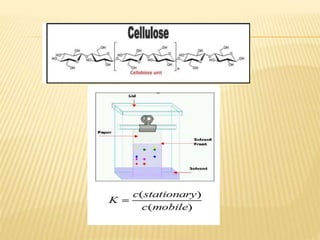
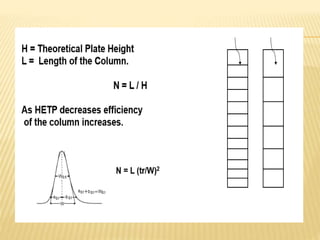
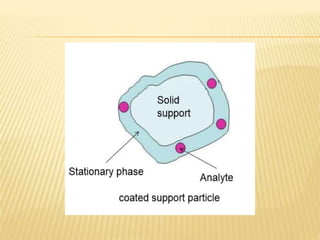
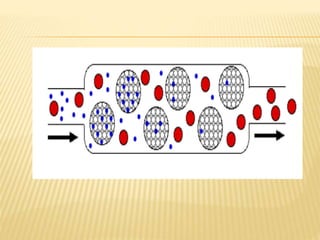
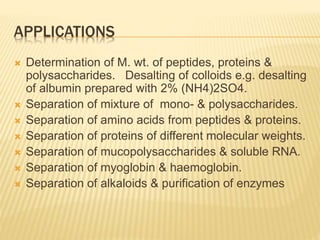
The document provides an overview of chromatography, a technique for separating the components of a mixture based on their distribution between a mobile phase and a stationary phase. It discusses the history of chromatography, various techniques such as thin layer, paper, column, partition, and gel permeation chromatography, highlighting their applications in science. Additionally, it references key contributions and sources related to chromatography.