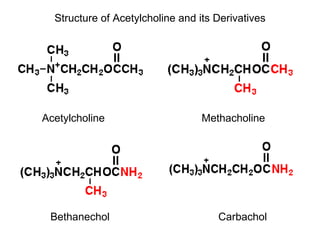
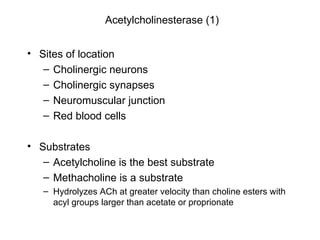
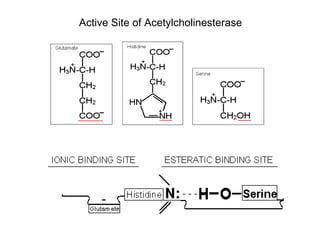
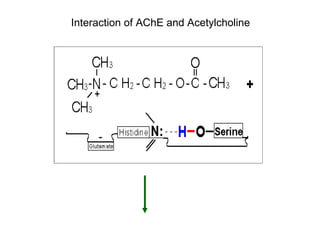
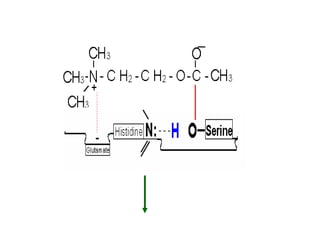
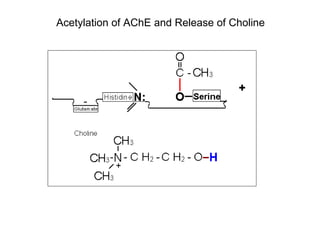
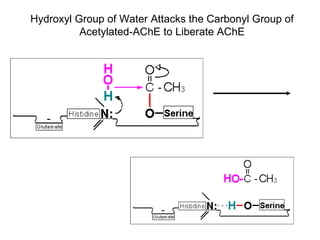
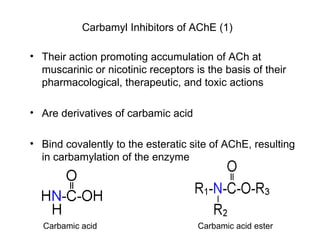
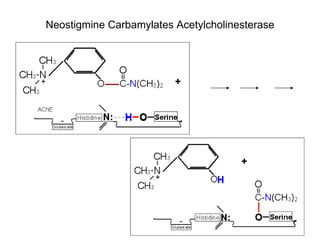
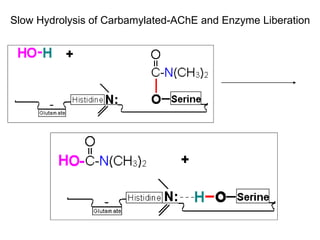
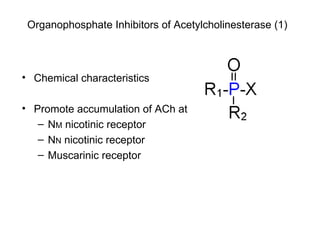
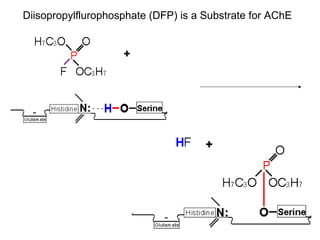
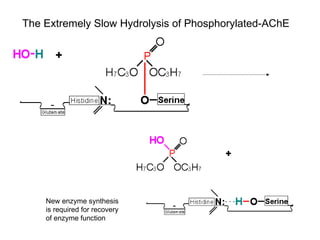
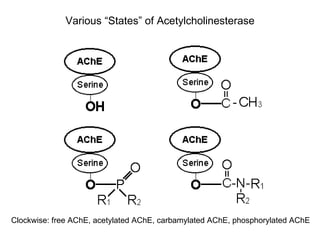
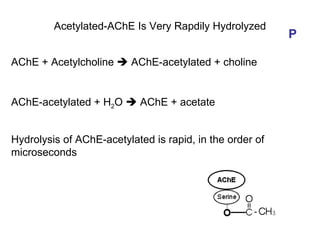
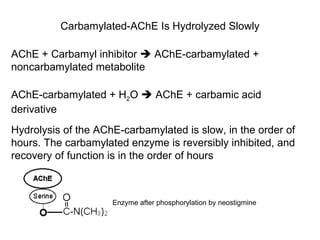
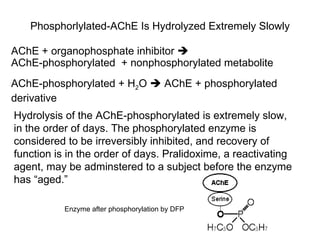
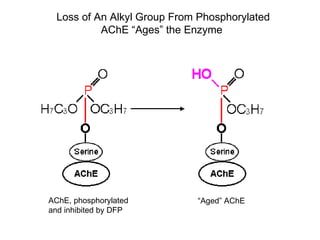
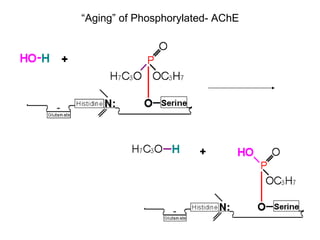
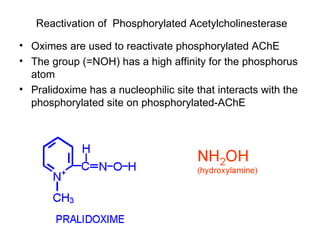
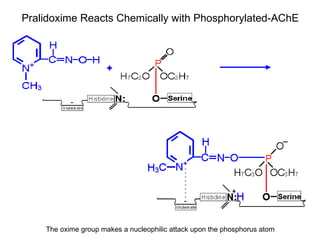
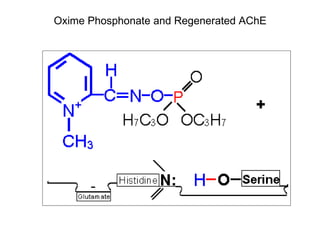
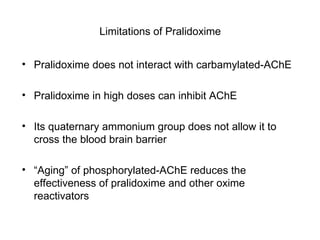
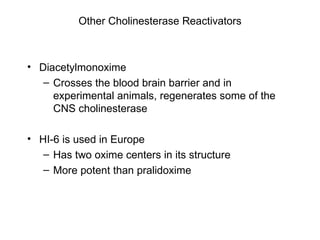
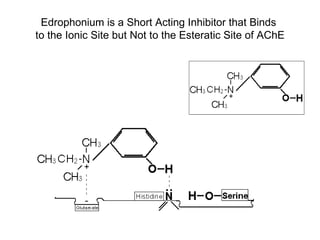
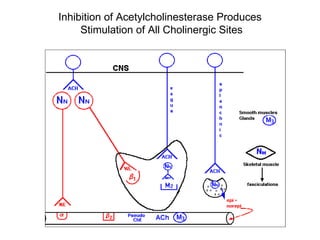
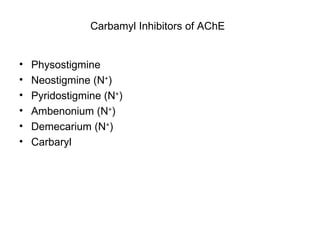
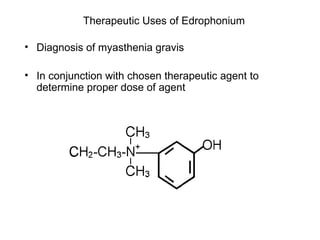
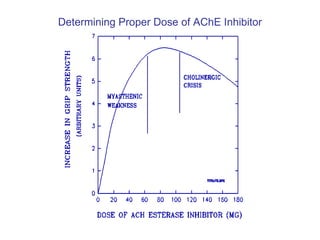
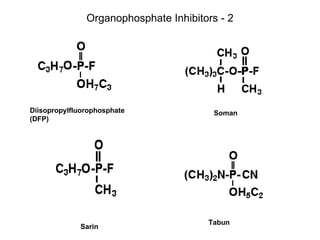
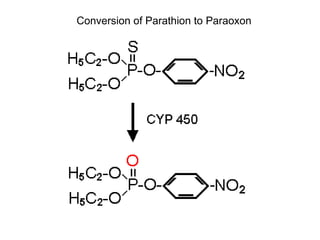
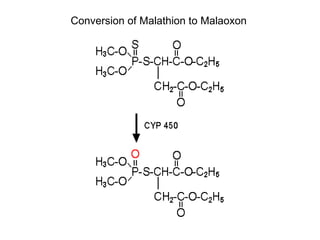
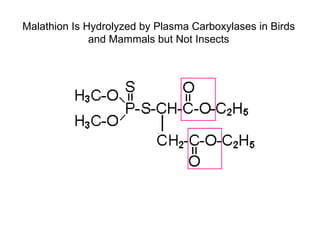
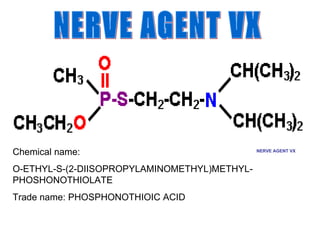
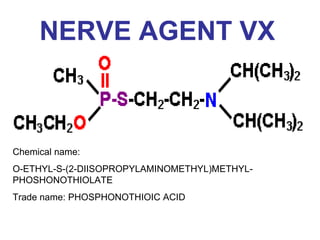
Cholinergic agents such as nicotine, muscarine, and pilocarpine act by mimicking acetylcholine at nicotinic and muscarinic sites. Acetylcholinesterase inhibitors such as neostigmine, pyridostigmine, and organophosphates promote the accumulation of acetylcholine by irreversibly or reversibly inhibiting the enzyme. These agents are used therapeutically for conditions like glaucoma but produce toxic effects in high doses by overstimulating cholinergic receptors. Pralidoxime can reactivate phosphorylated acetylcholinesterase and reverse some effects of organophosphate poisoning.