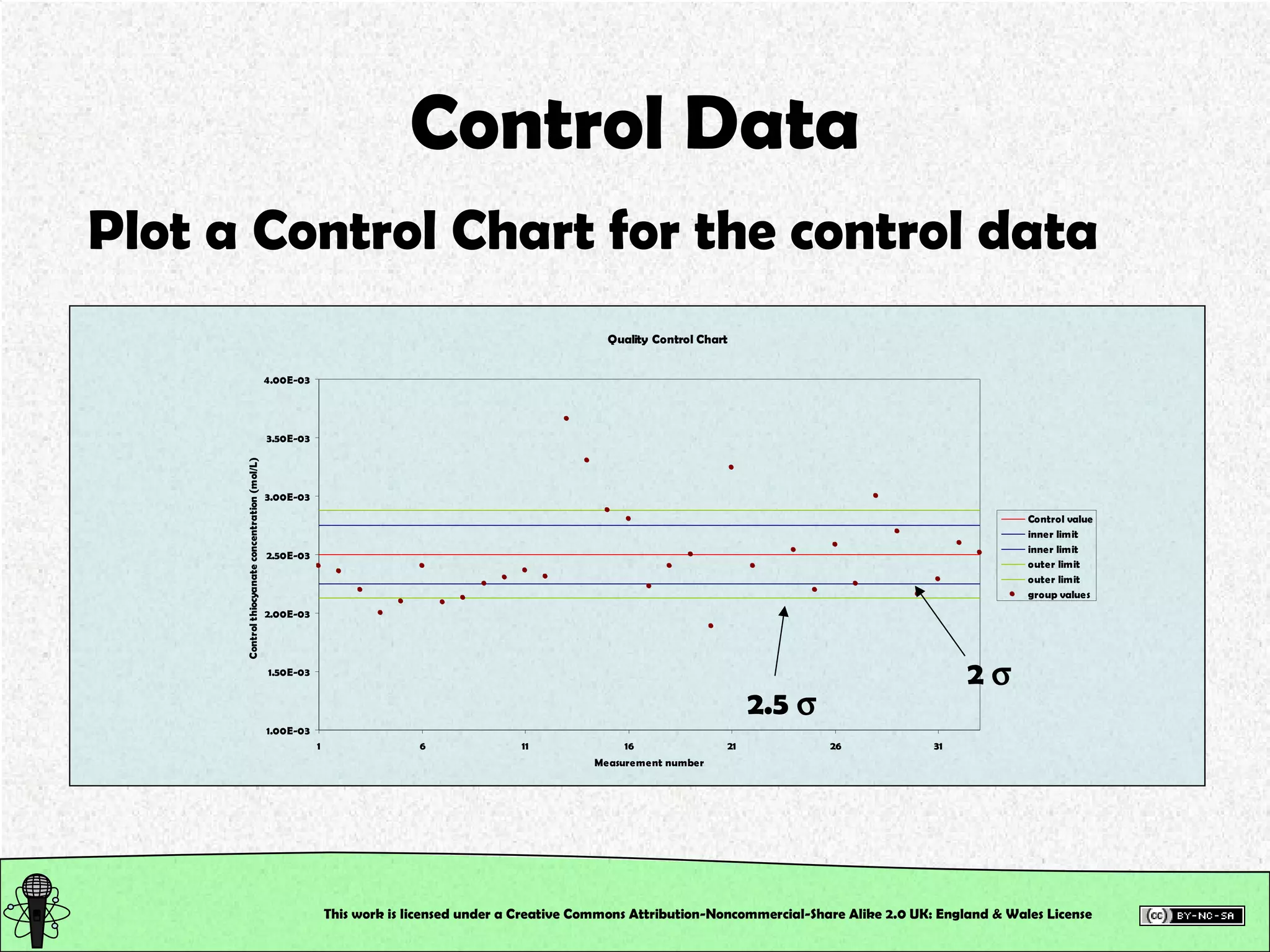
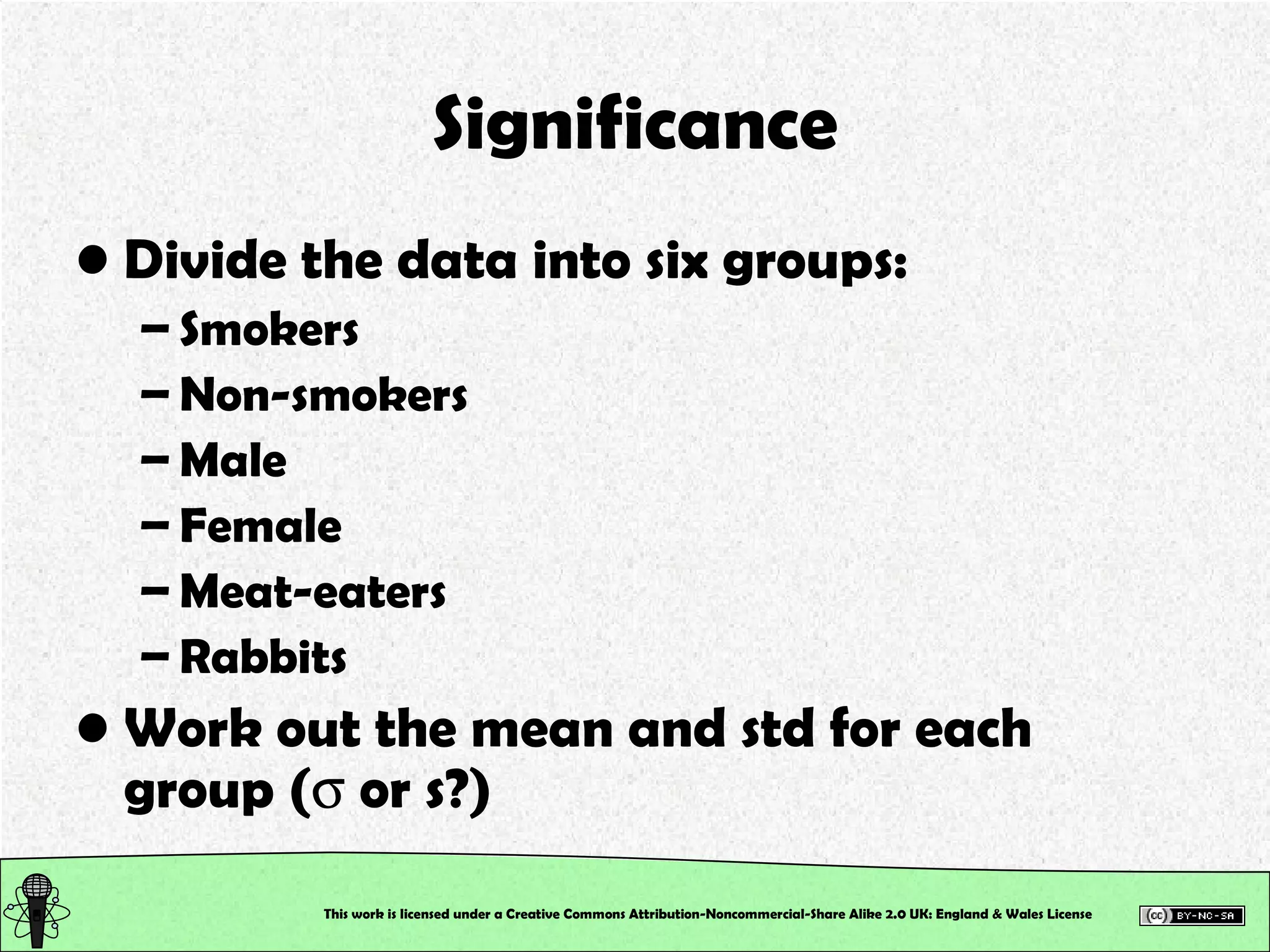
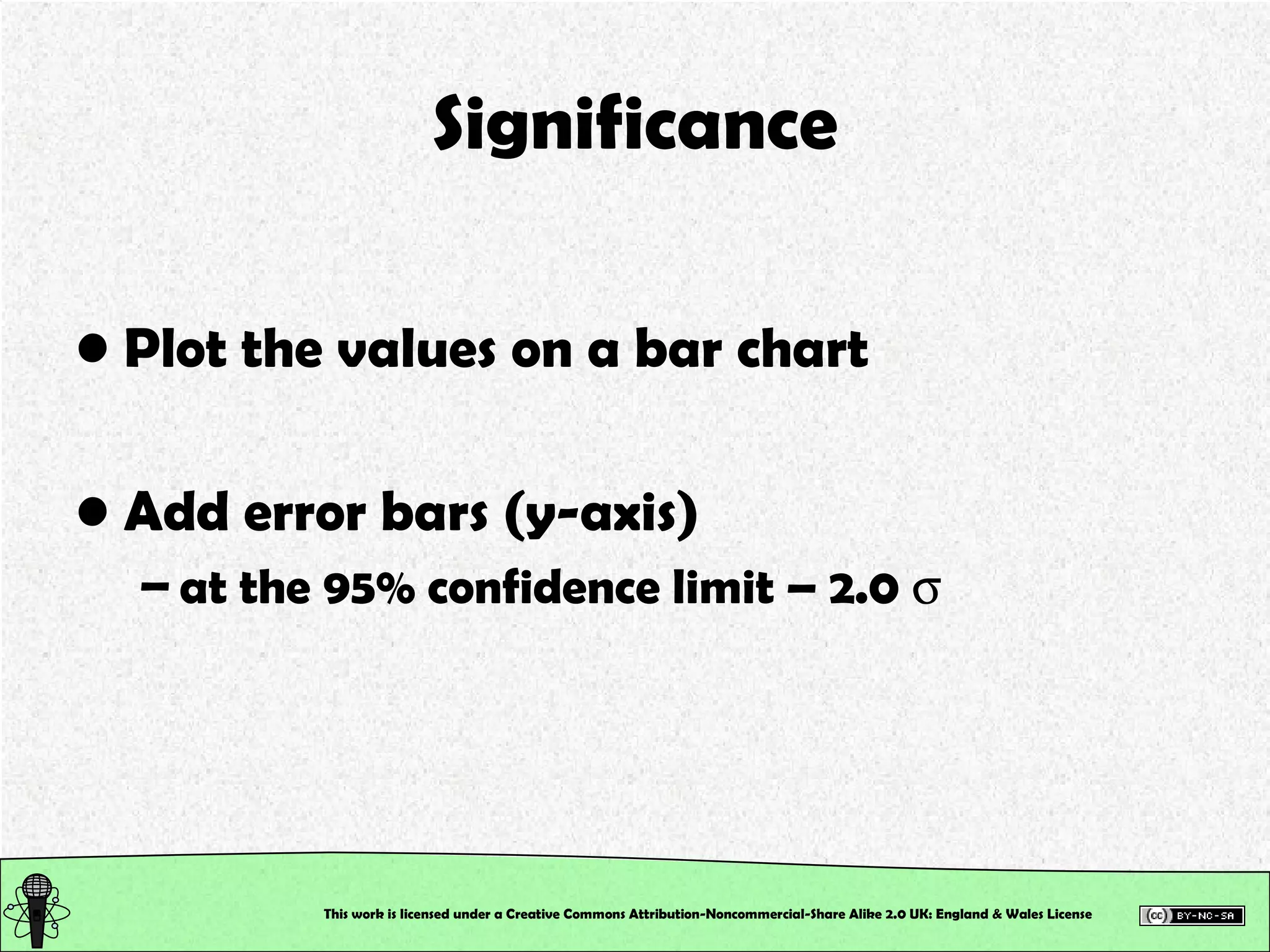
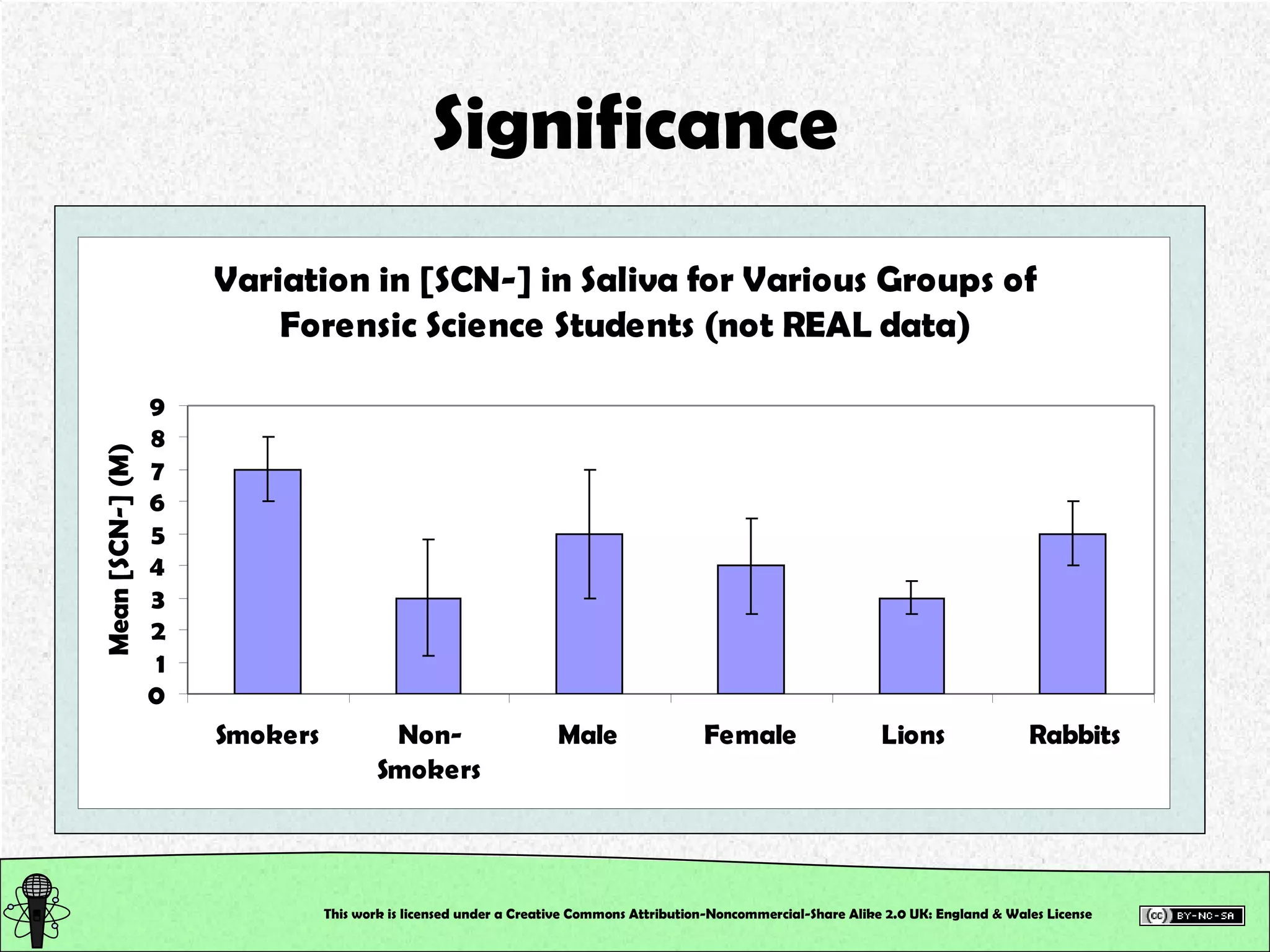
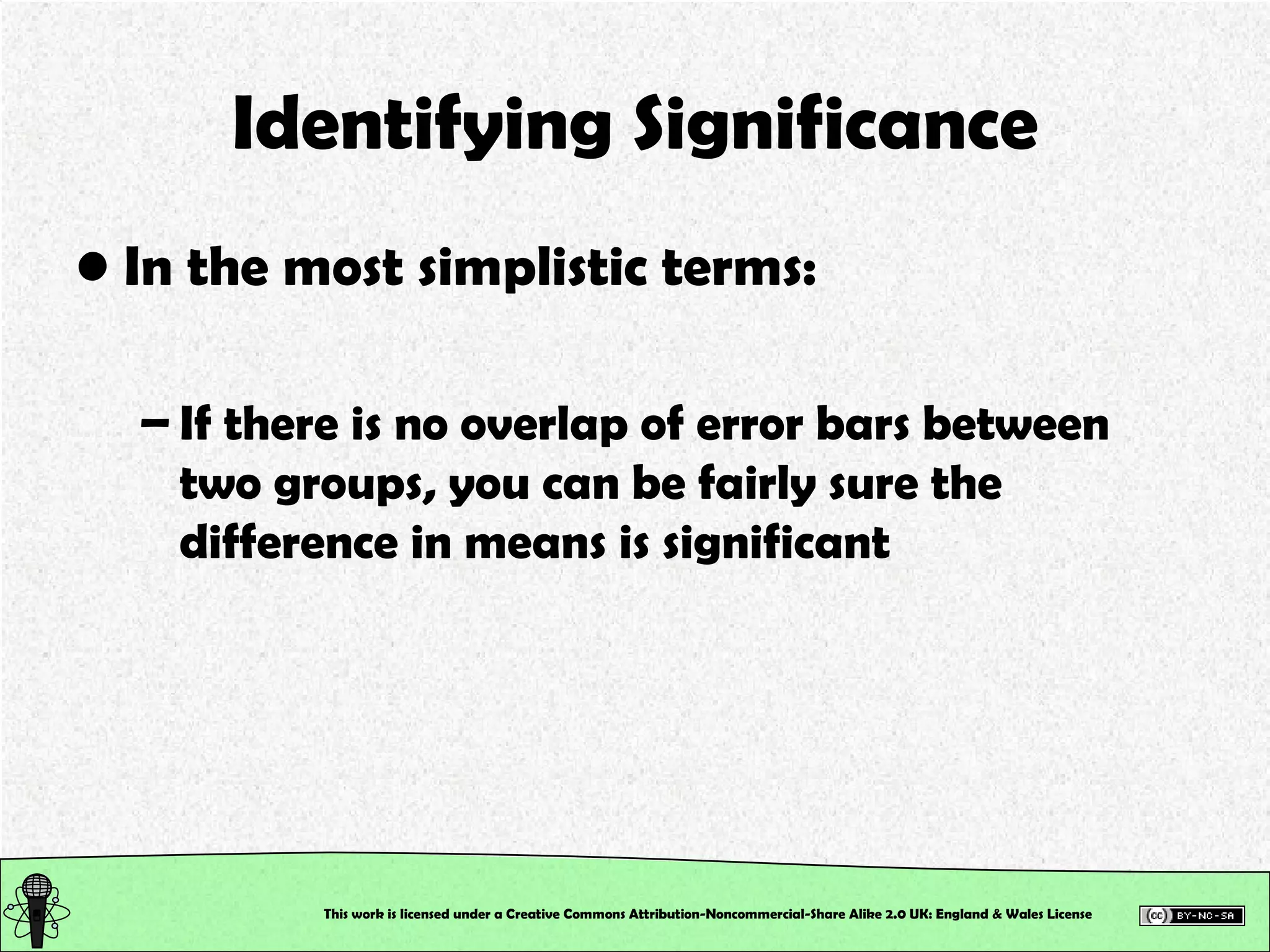
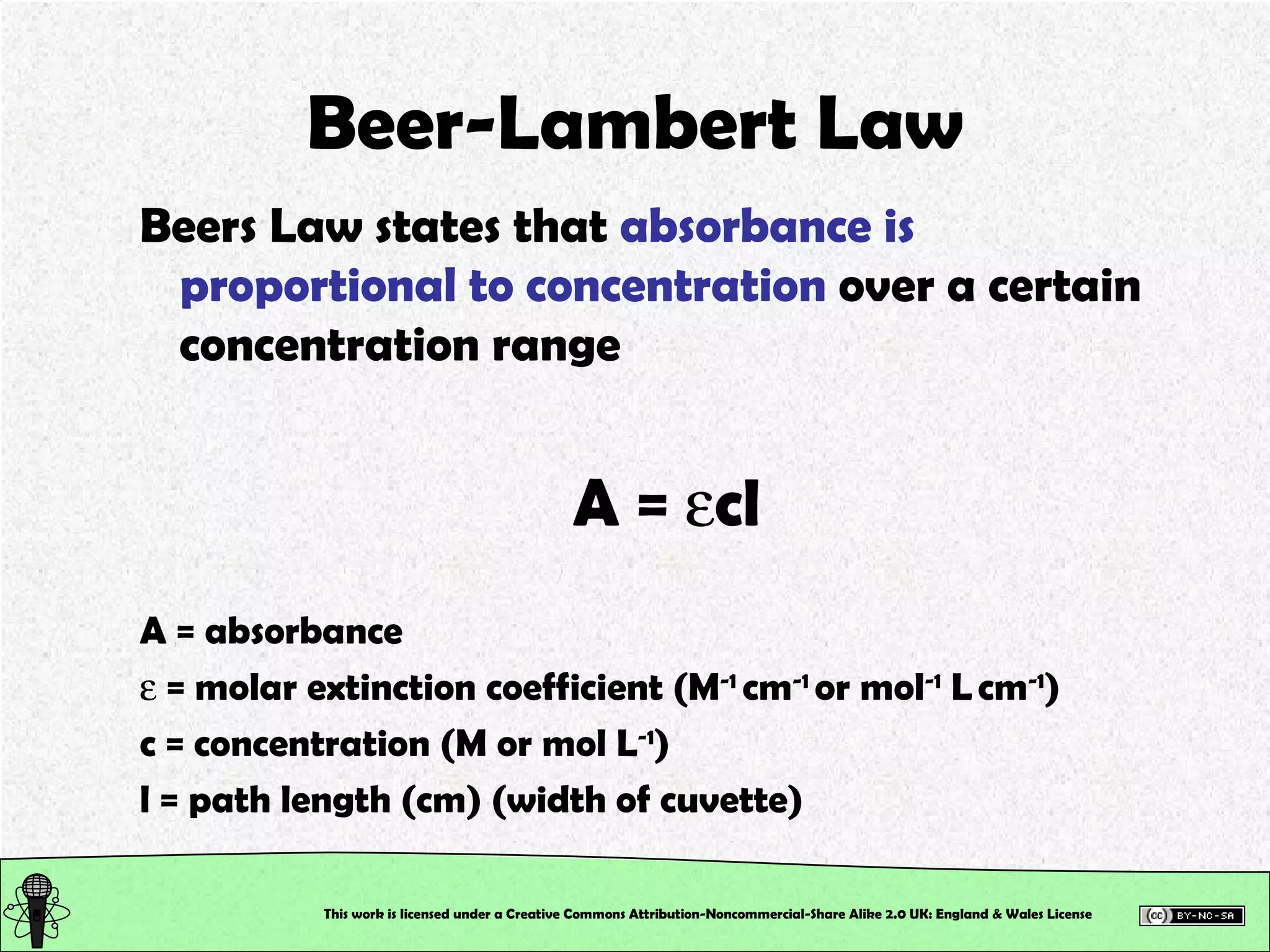
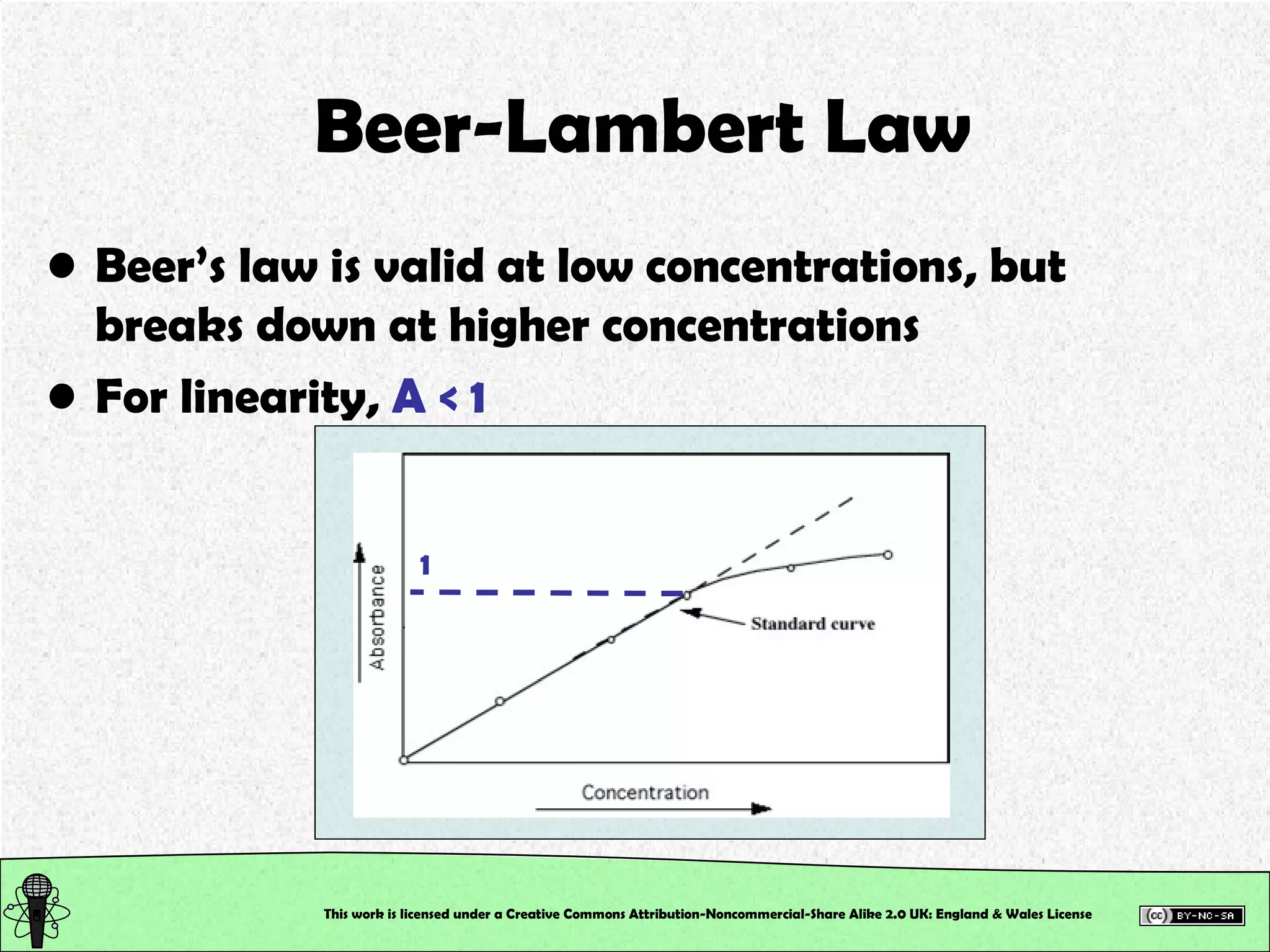
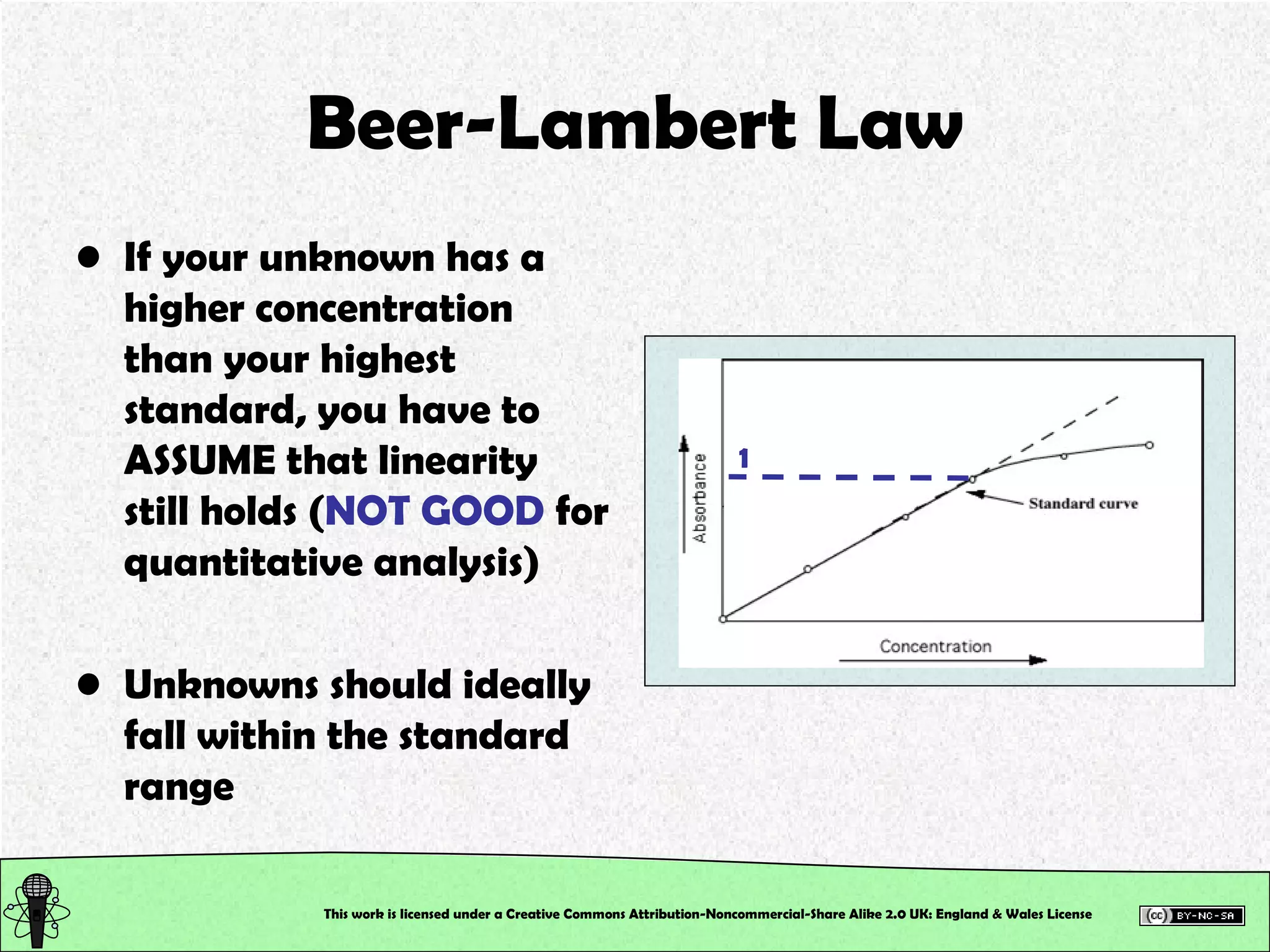
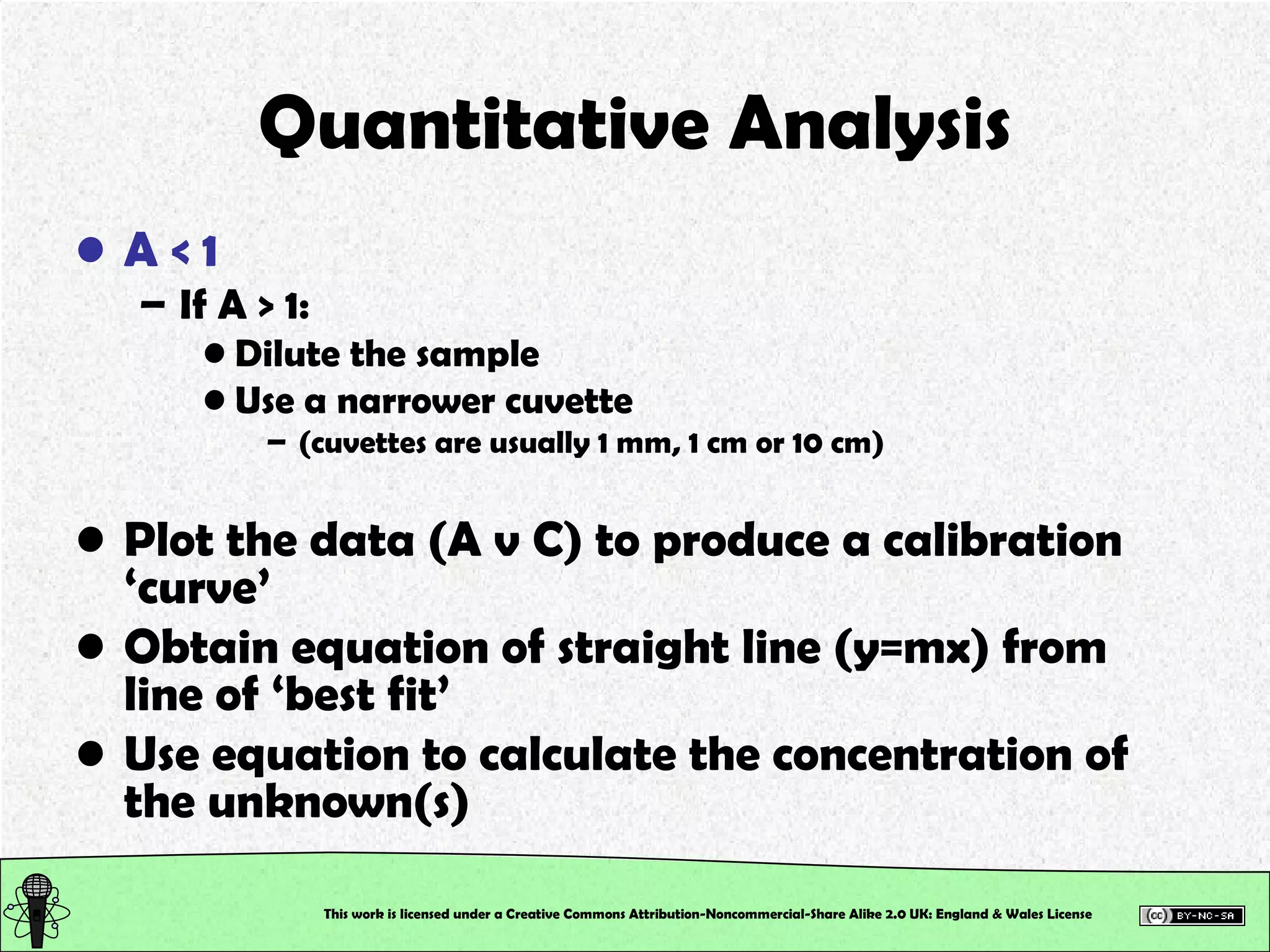
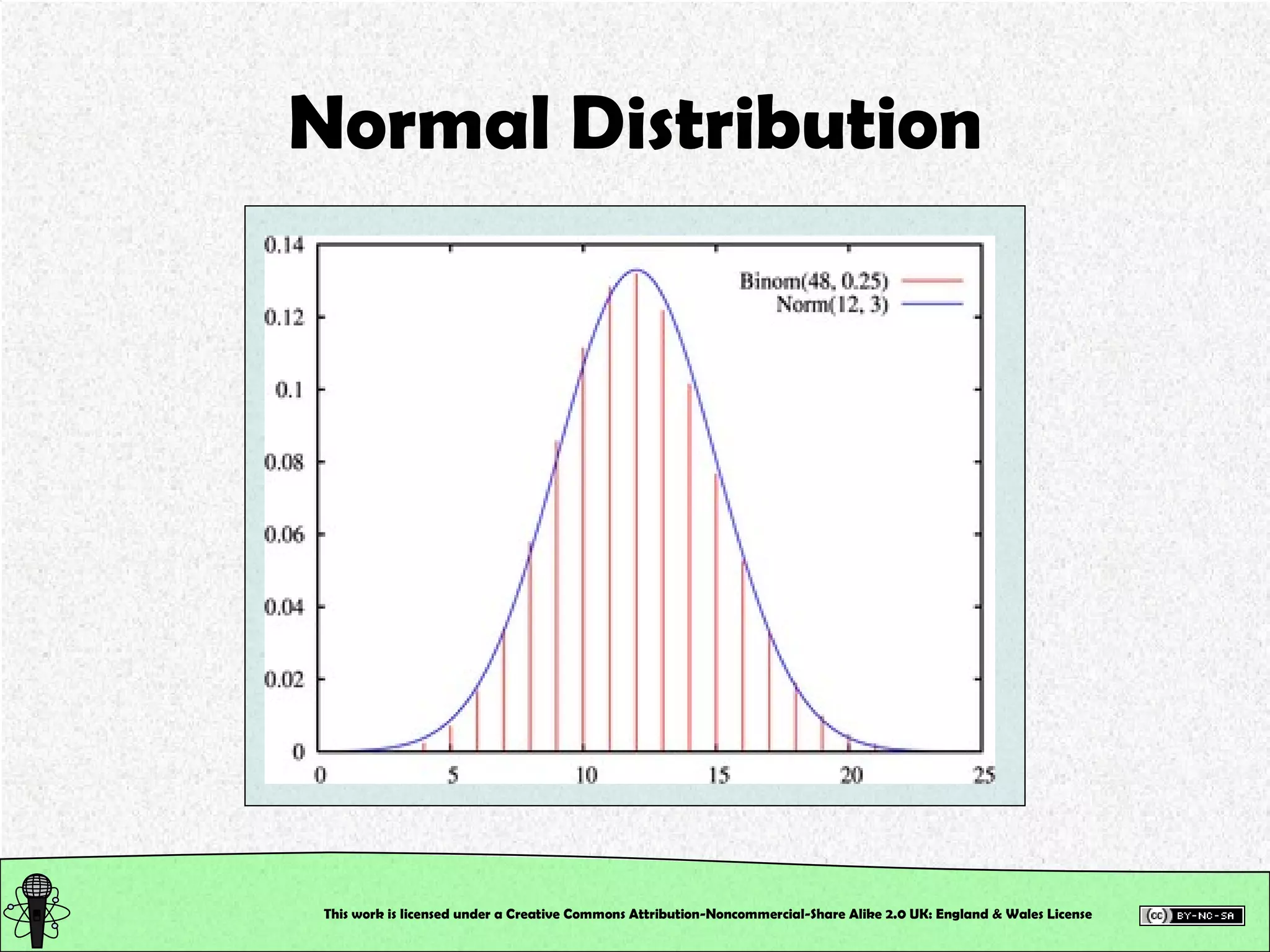
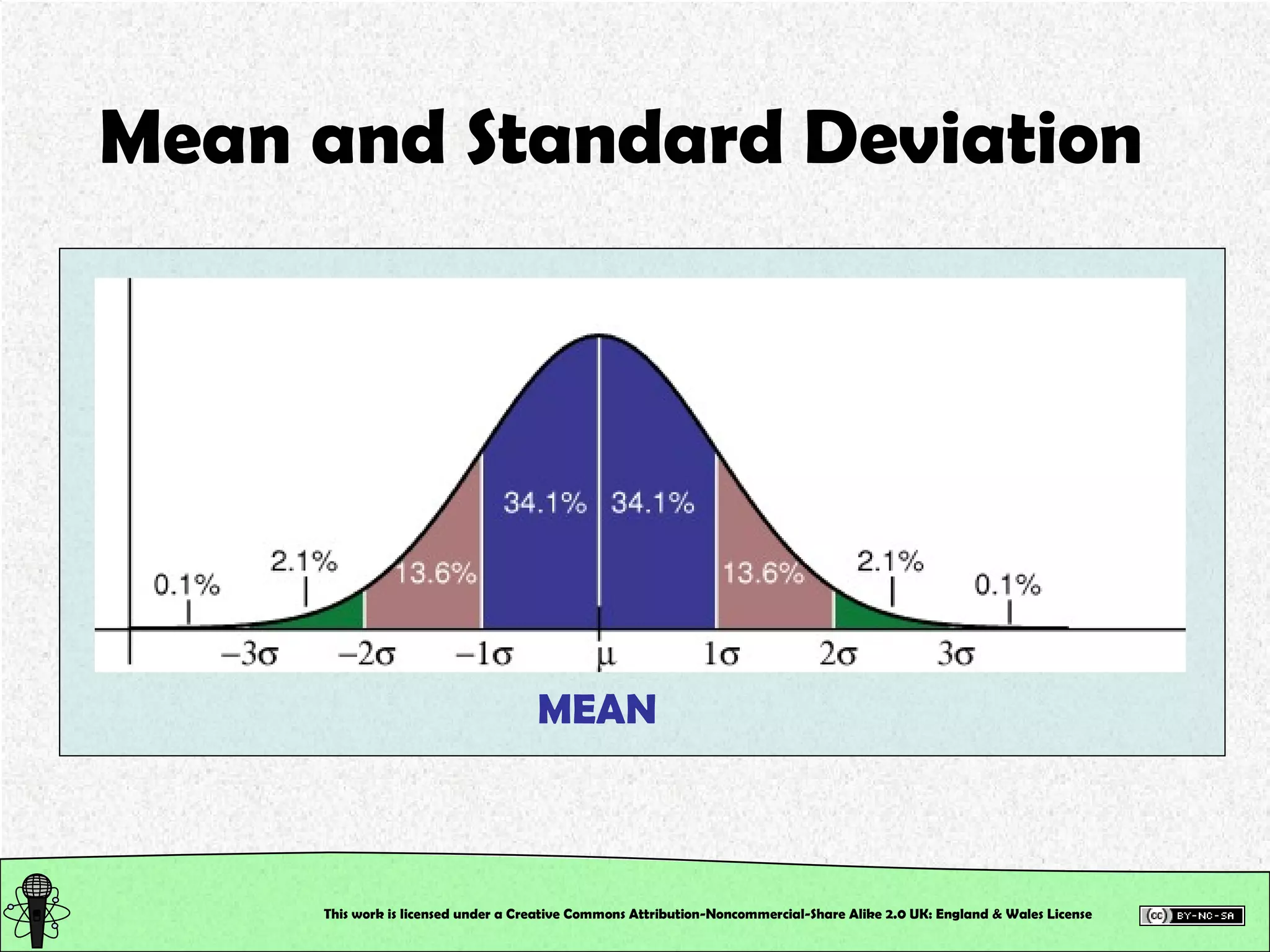
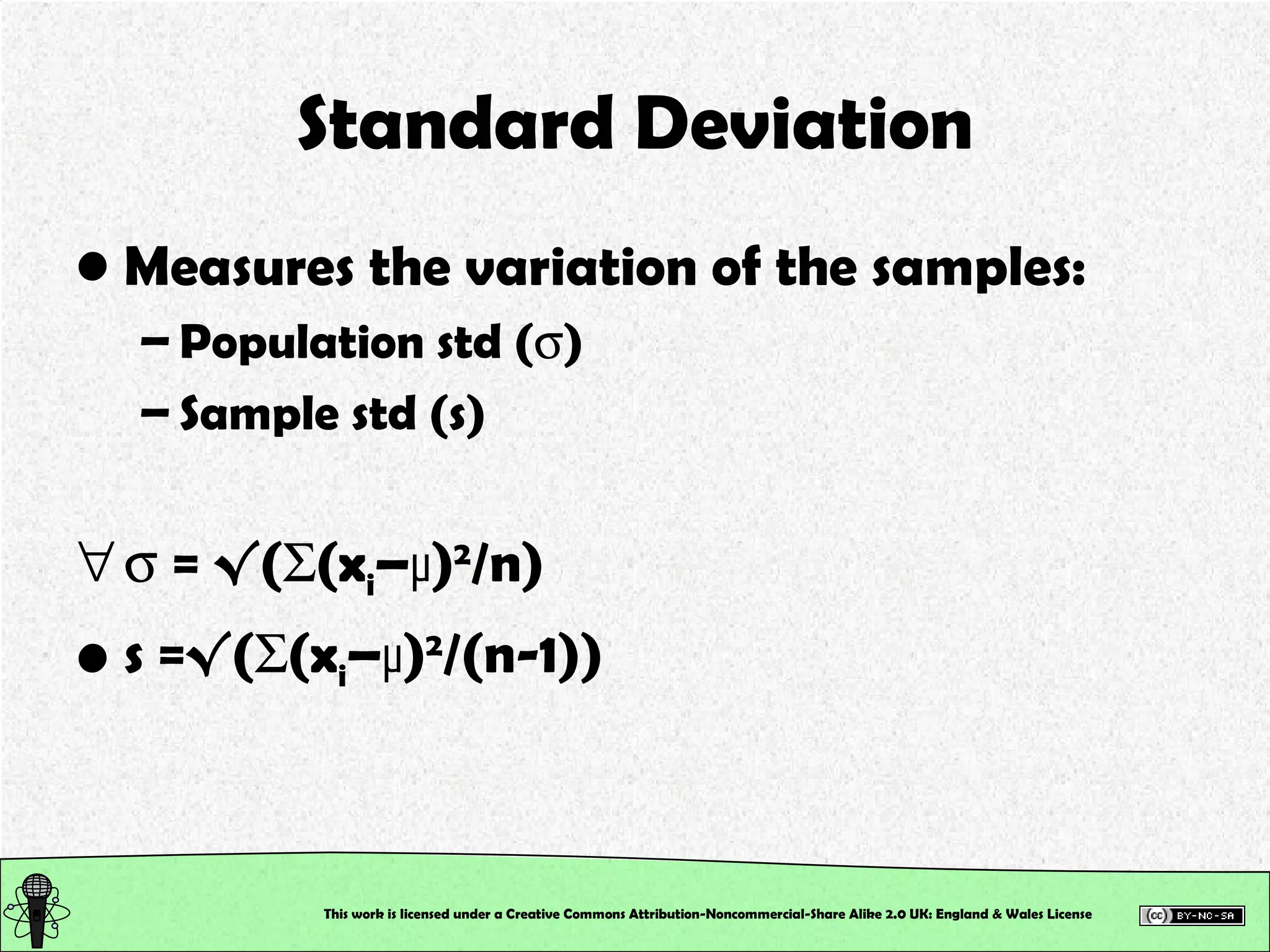
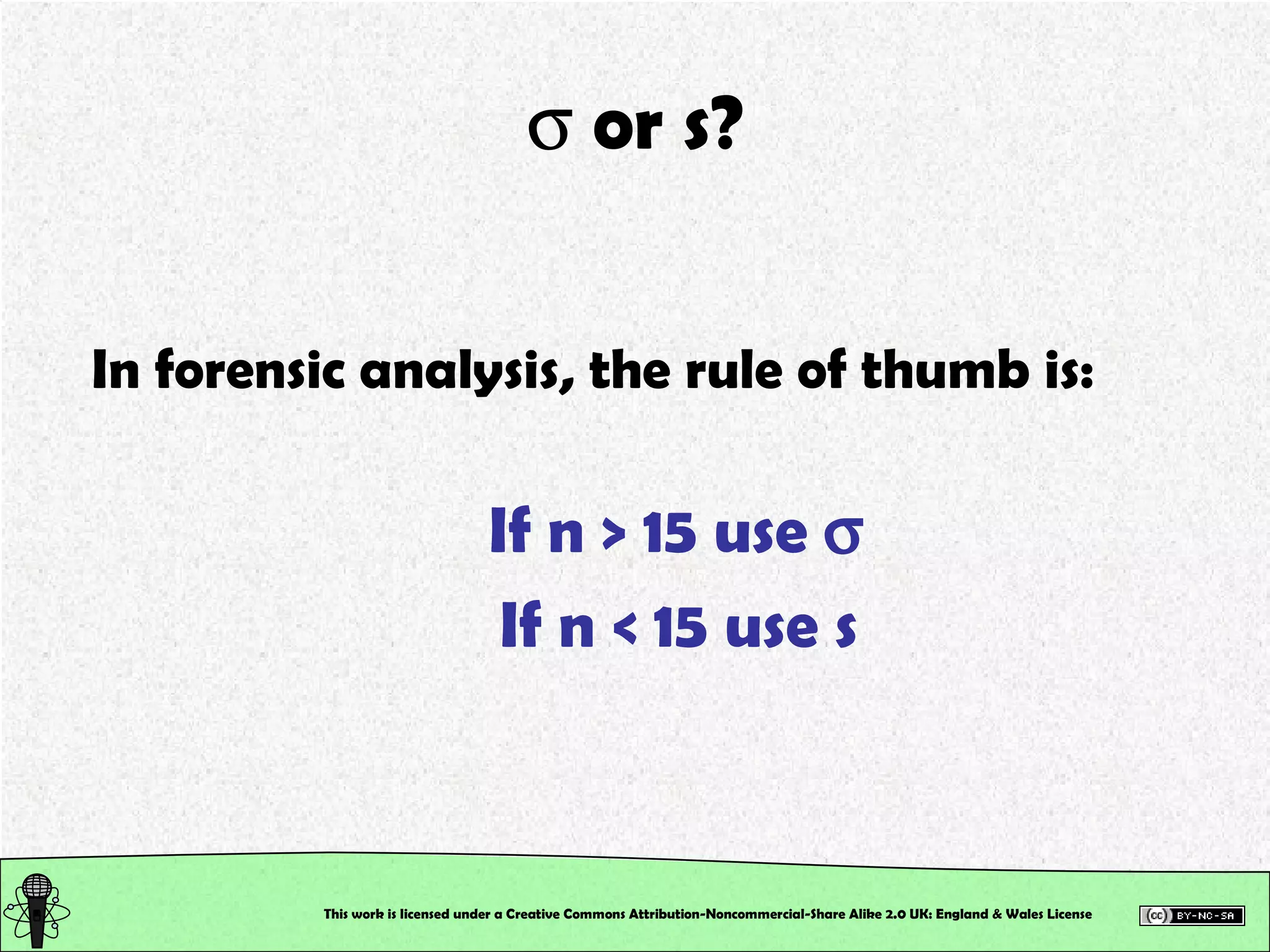
This document outlines a practical session at the University of Lincoln focused on determining thiocyanate (SCN-) levels in saliva using the Beer-Lambert law for colorimetric analysis. Key topics include data types, calibration curves, statistical analysis, control charts, and significance testing. The overall aim is to educate students on quantitative methods in chemistry and how to analyze and interpret their results accurately.


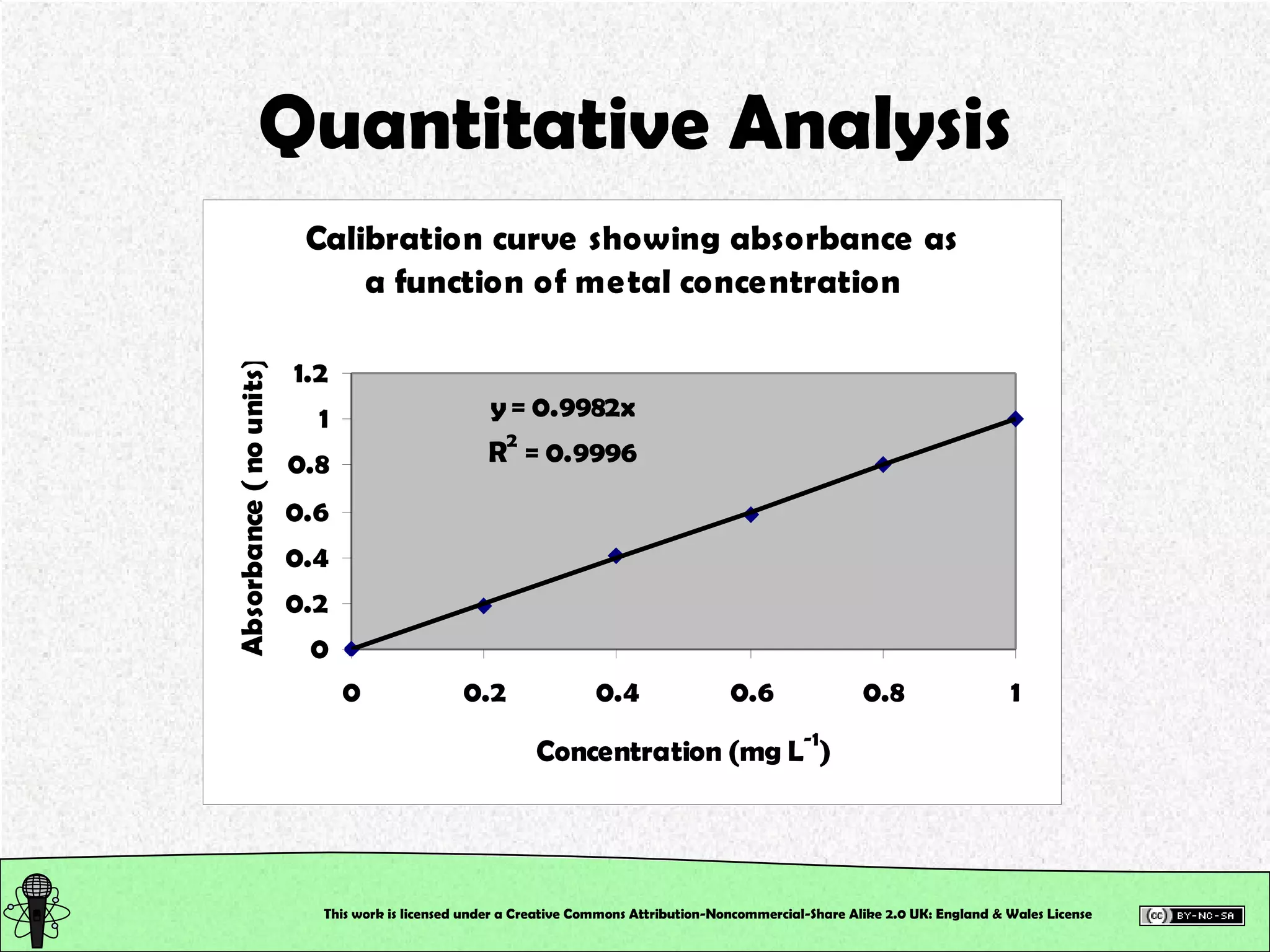
![The Practical Determine the thiocyanate (SCN - ) in a sample of your saliva using a colourimetric method of analysis Calibration curve to determine the [SCN - ] of the unknowns This was ALL completed in the practical class Some of your absorbance values may have been higher than the absorbance values of your top standards… is this a problem???? This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.0 UK: England & Wales License](https://image.slidesharecdn.com/pre-labsalivapracticaledited-100924023840-phpapp02/75/Chemical-and-Physical-Properties-Practical-Session-3-2048.jpg)

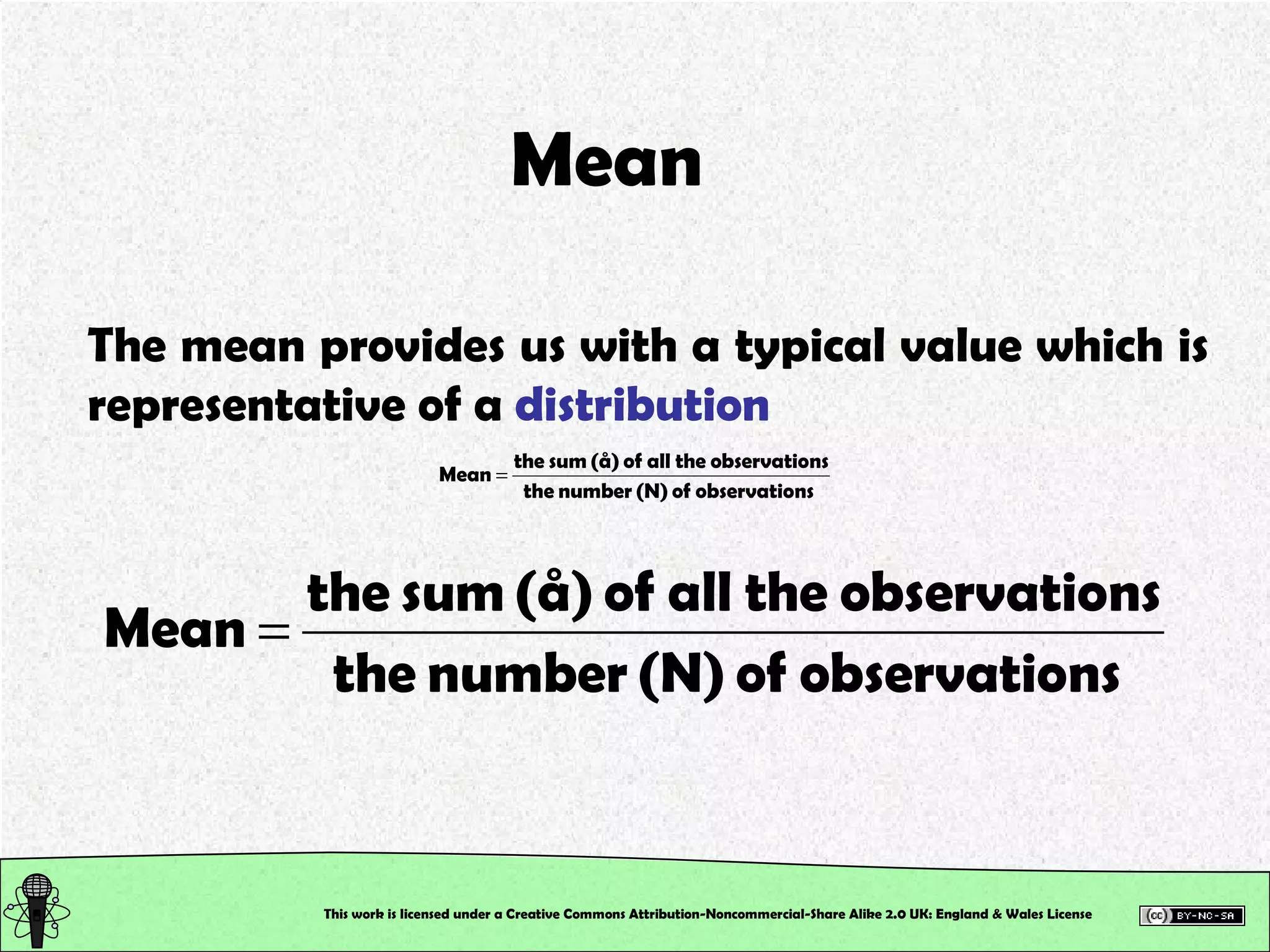
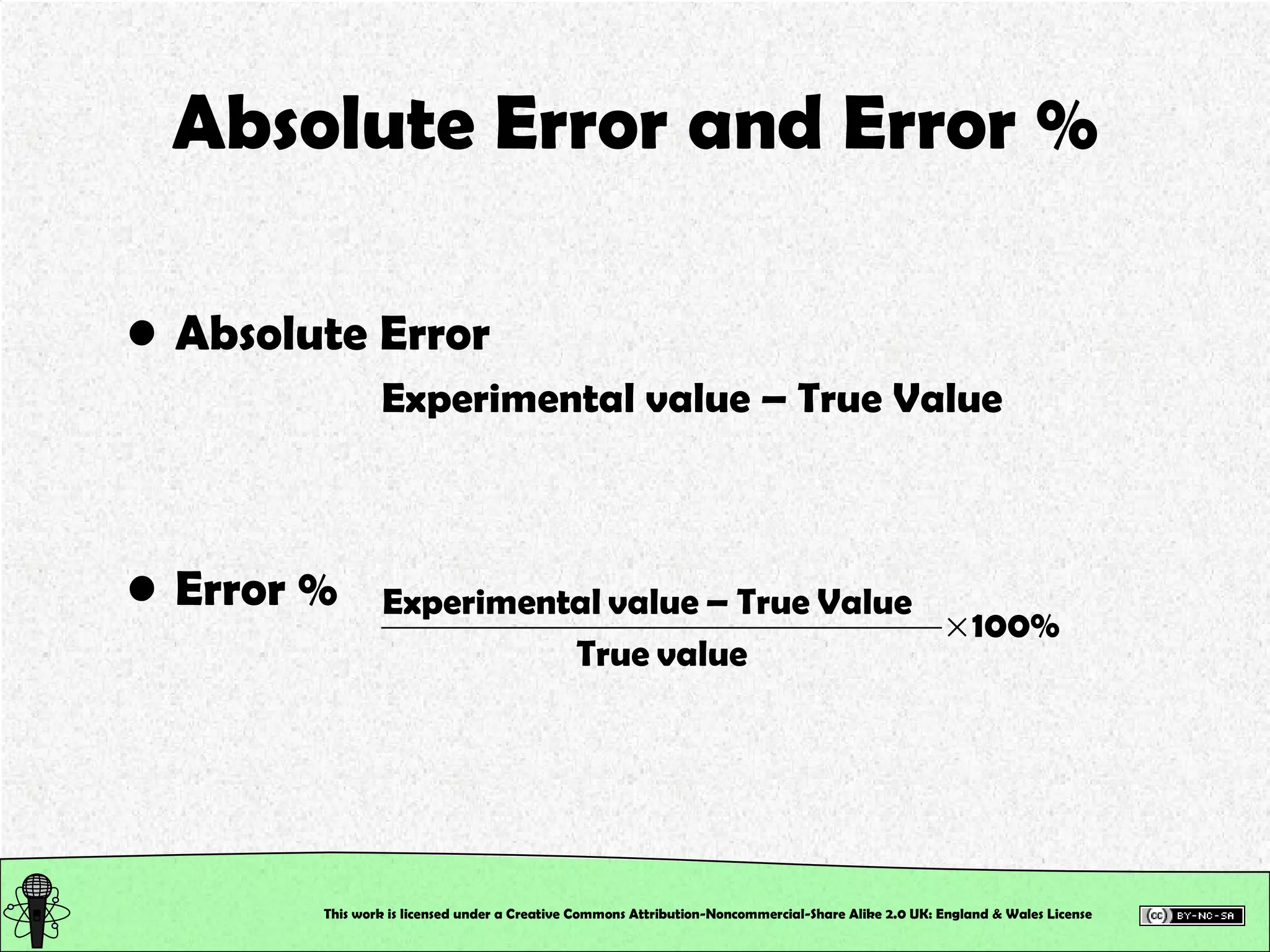
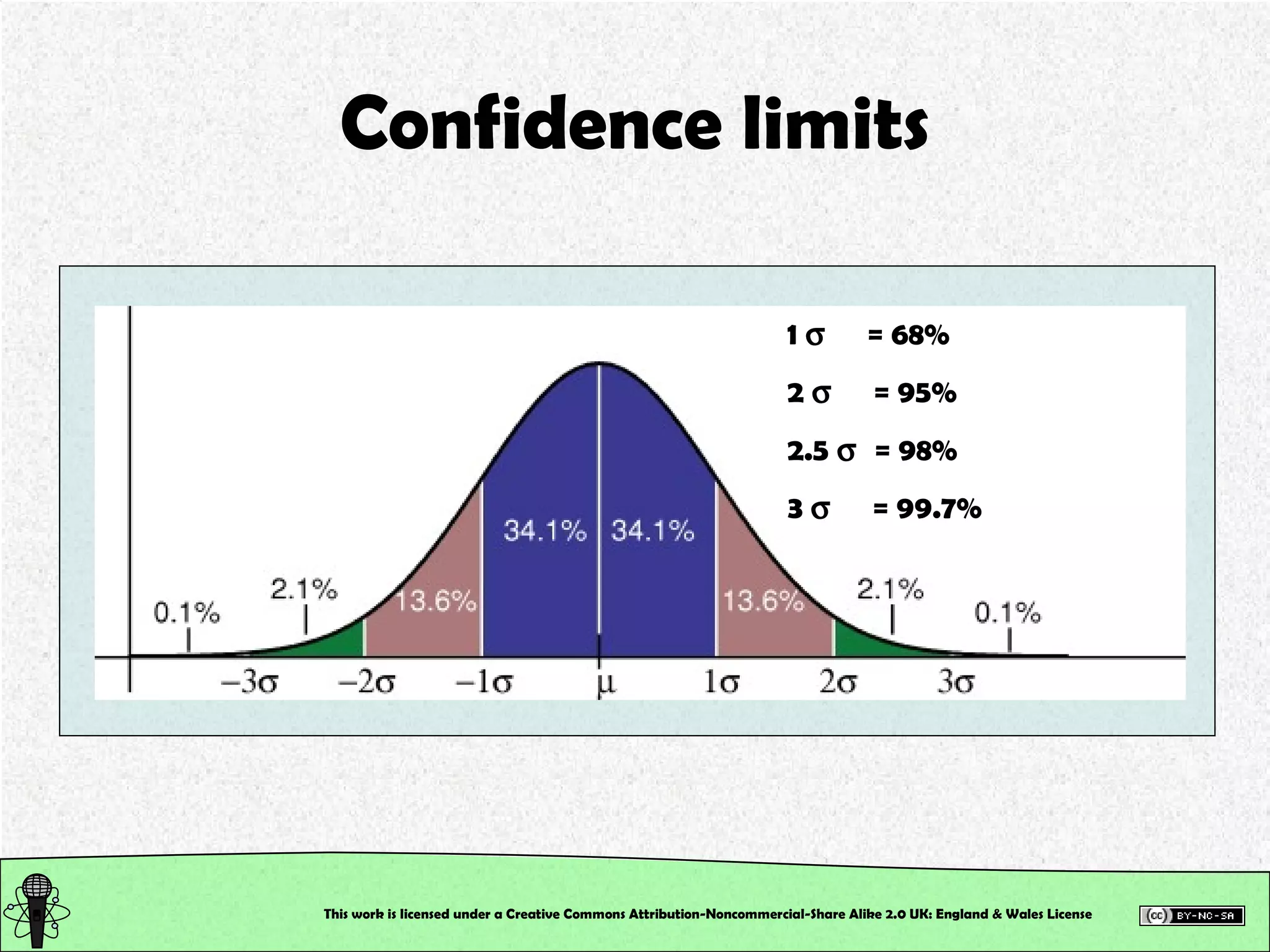
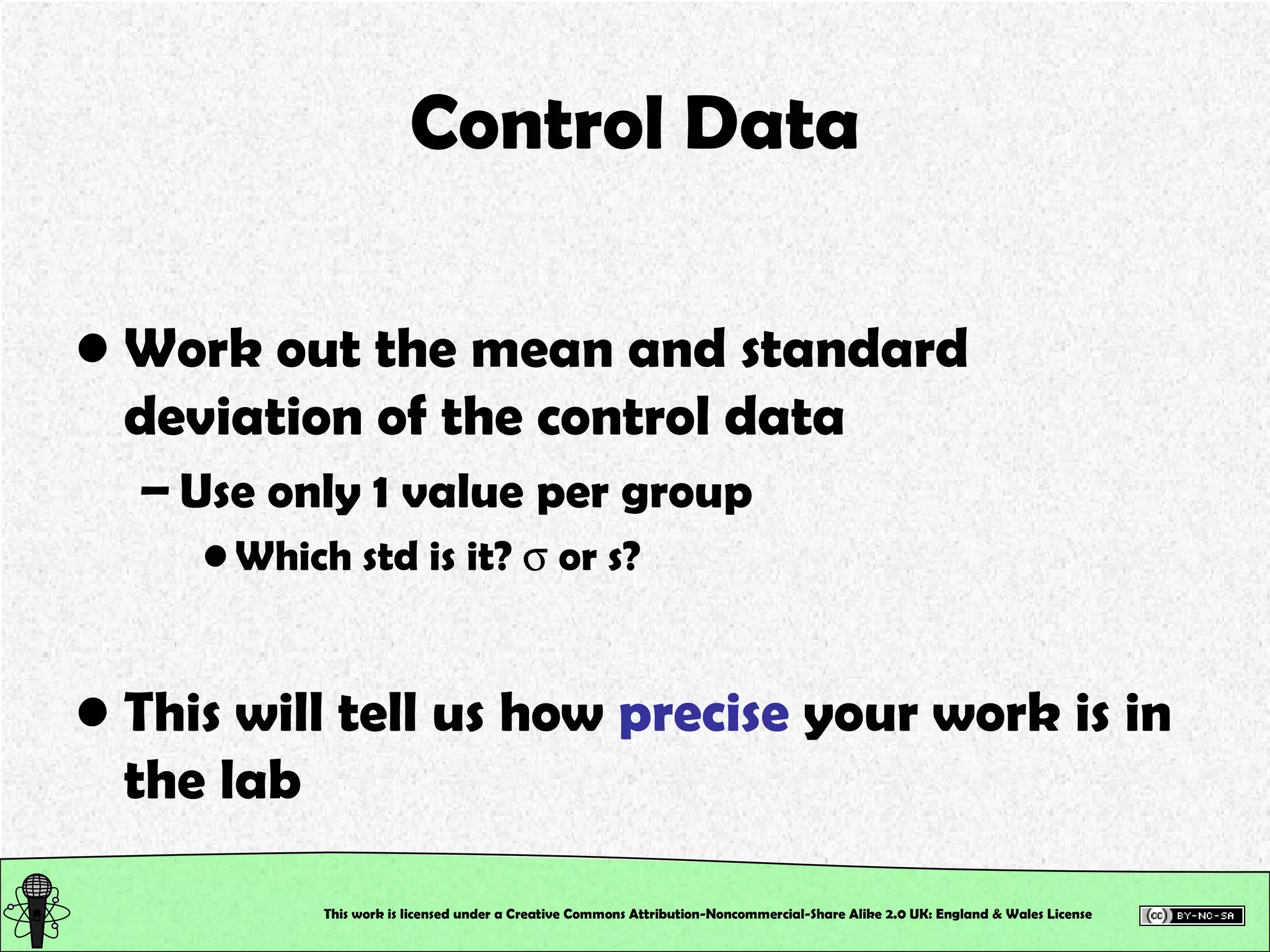
![Control Data Calculate the Absolute Error and the Error % True value of [SCN – ] in the control = 2.0 x 10 –3 M This will tell us how accurately you work, and hence how good your calibration is!!! This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 2.0 UK: England & Wales License](https://image.slidesharecdn.com/pre-labsalivapracticaledited-100924023840-phpapp02/75/Chemical-and-Physical-Properties-Practical-Session-19-2048.jpg)