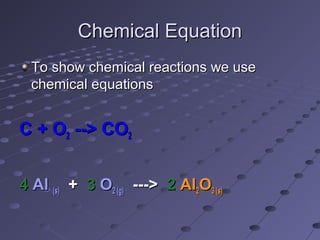
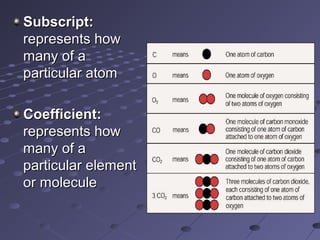
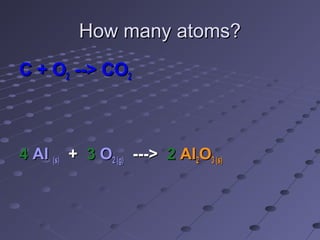
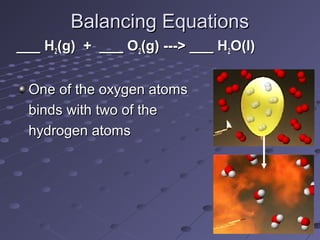
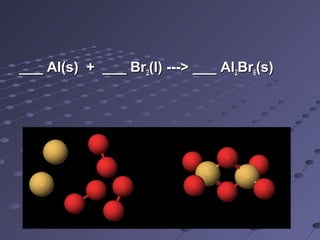
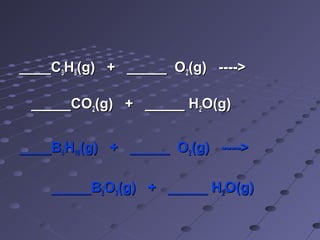
The document outlines an agenda for a chemistry class that includes balancing chemical equations. It provides examples of unbalanced equations and steps for balancing equations by changing coefficients while keeping subscripts the same. The objectives are to explain the difference between elemental and chemical formulas, recognize parts of equations, describe how compounds differ from their components, and balance equations.