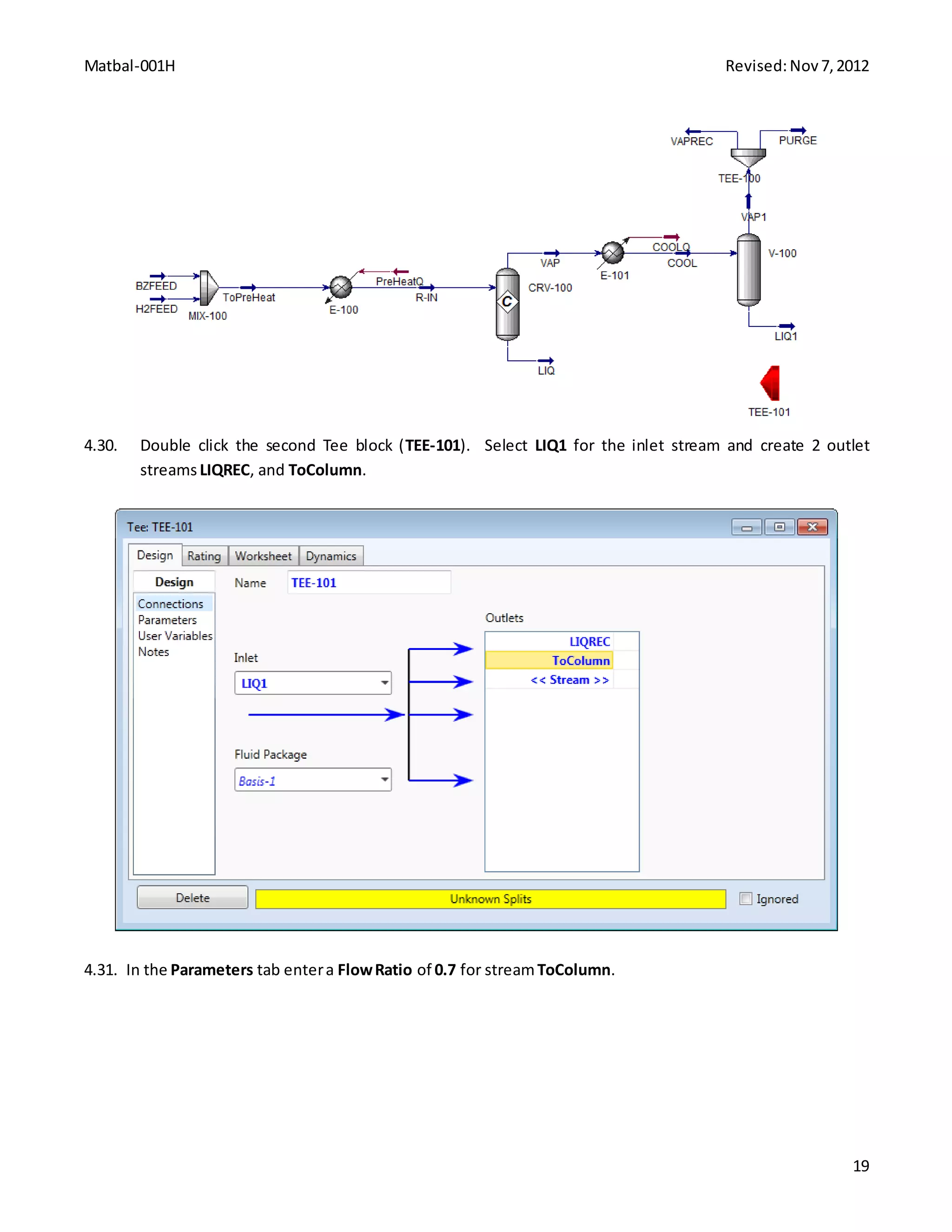
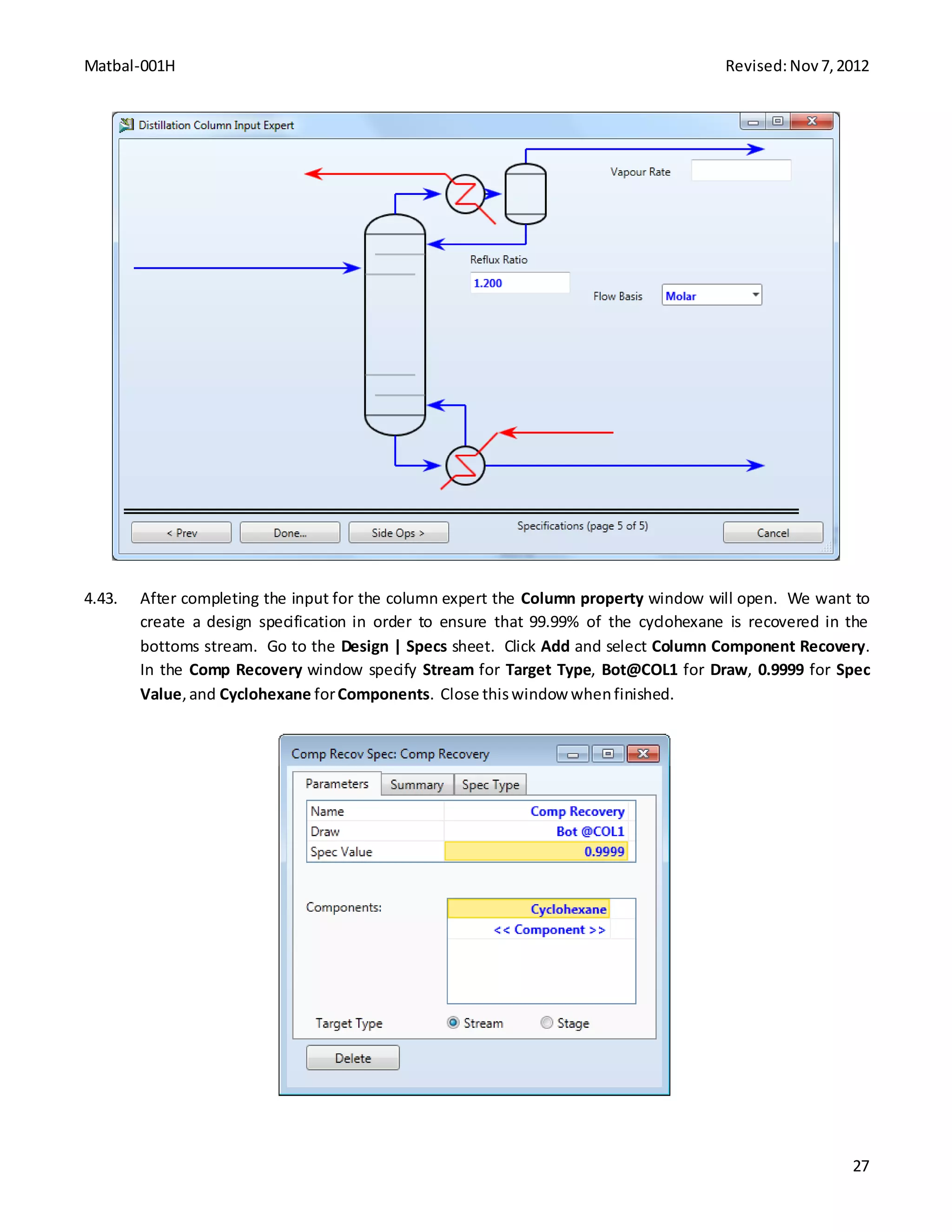
This document provides instructions for constructing an Aspen HYSYS simulation of cyclohexane production via benzene hydrogenation. It includes process details like feed streams, operating specifications, and reaction chemistry. The simulation involves building a flowsheet with a mixer, heater, conversion reactor, cooler, separator, recycle loops, and distillation column. Step-by-step instructions are provided to define each unit operation using the HYSYS interface and solve the simulation.




















































































































































































































































































































































































































































































































![Dyn-005H Revised:Nov16,2012
1
Controller Parameter Tuning with Aspen HYSYS® V8.0
1. Lesson Objective:
Use Ziegler-Nichols, Cohen-Coon, and time integral tuning methods to determine the optimal
controllertuningparameters
To understandbasicsof dynamicsimulation inAspenHYSYS
2. Prerequisites
Aspen HYSYS V8.0
File Dyn-005H_Controller_Tuning_Start.hsc
3. Background
There are several methodsfortuningacontroller,includingZiegler-Nichols,Cohen-Coon,andthe ITAEtuning
method.Inthistutorial,we will utilizethesemethodstodeterminethe tuningparametersfora secondorder
system. Most processescanbe well approximatedbyafirstorderresponse withtime delay. Analysisof this
response canthenbe usedto determine tuningparametersforthe process.
A processreactioncurve can be obtainedfromthe controlledprocesswith the controllerdisconnected. From
the processreactioncurve,one can acquire valuesof K,τ, andα, whichallowsapproximationof the process
reactioncurve viaa first-ordersystemwithtime delay:
( )
Giventhis, several tuningmethodscanbe used toobtainapproximate tuningparameters.Inthistutorial,we will
use a PID controller,whichhasthe following tuningrules:
Tuning Method
Ziegler-Nichols
( )
Cohen-Coon ( ) ( ( ))
[
( )
( )
] [
( )
]
ITAE
( )
[ ( )]
( )](https://image.slidesharecdn.com/chemicalengineeringsimulationsinaspe1-160802010606/75/Chemical-engineering-simulations-503-2048.jpg)


![Dyn-005H Revised:Nov16,2012
4
Usingthe Zieglar-NicholsPIDtuningrules we canobtainthe followingtuningparameters:
( )
( )
( )
Usingthe Cohen-CoonPIDtuningruleswe canobtainthe followingtuningparameters:
( )( ( ))
[
( )
( )
]
[
( )
]
Usingthe MinimumITAEPID tuningruleswe canobtainthe followingtuning parameters:
( )
[ ( )]
( ) ( )
We can thenuse a simulatorsuchas AspenHYSYS to model the processanddeterminethe optimal tuning
parametersforthissystem.
Aspen HYSYS Solution
4.01. Start AspenHYSYS V8.0. OpenDyn-005_Controller_Tuning_Start.hsc.
4.02. On the face plate for the temperature controller(TIC-100),selectAutotoactivate the controller.](https://image.slidesharecdn.com/chemicalengineeringsimulationsinaspe1-160802010606/75/Chemical-engineering-simulations-506-2048.jpg)



































































































