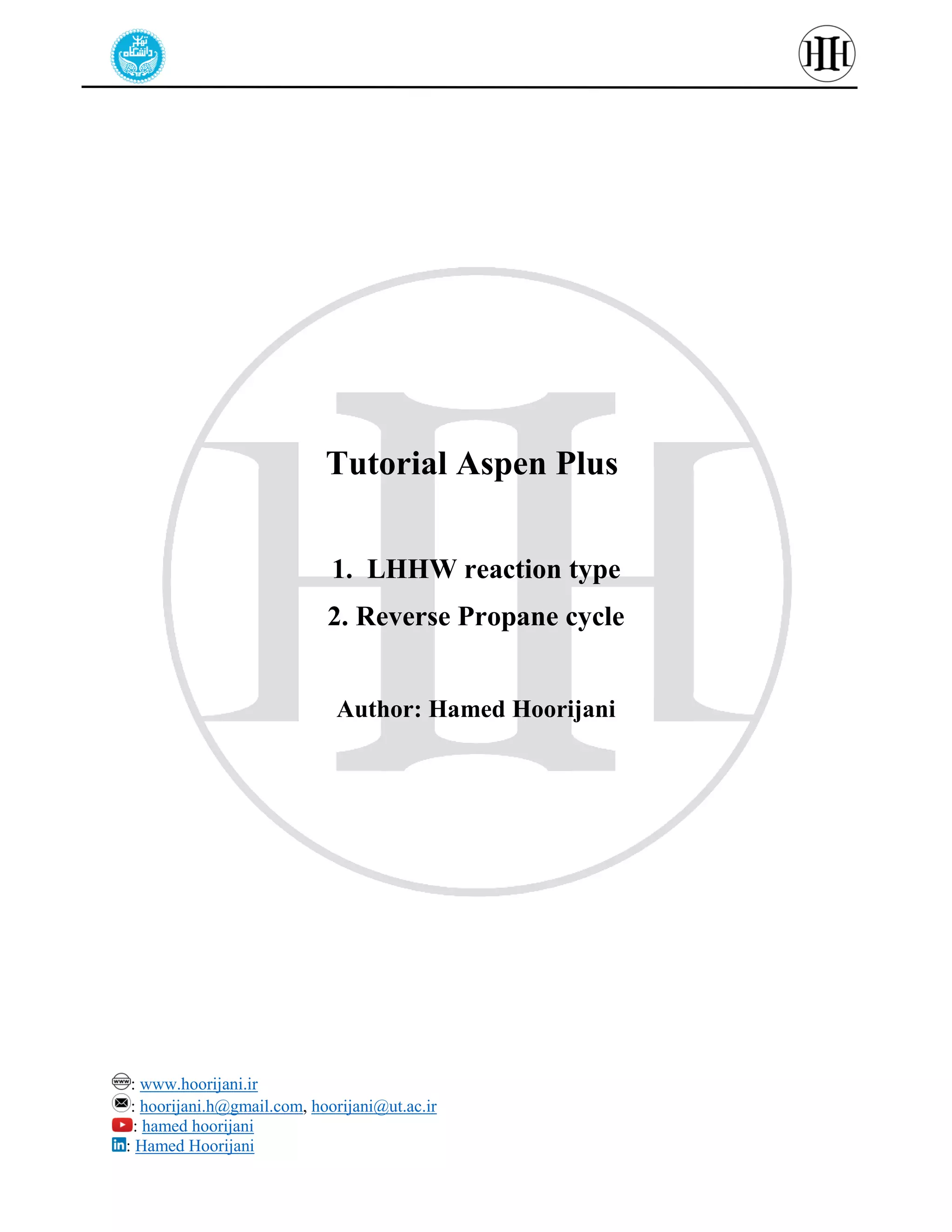
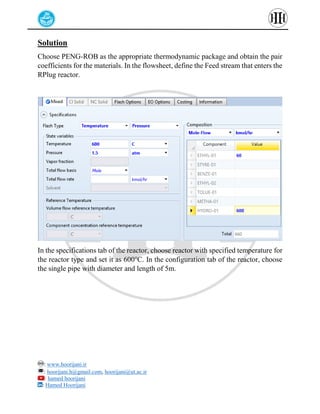
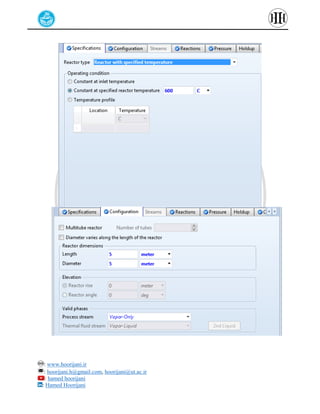
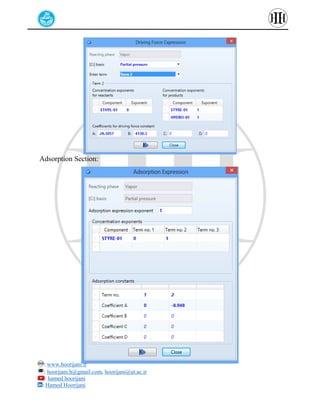
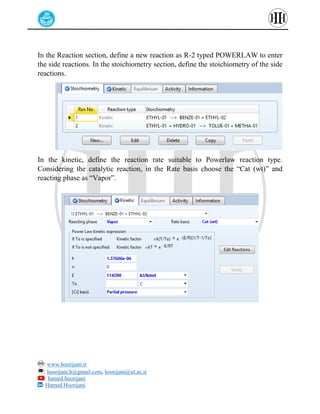
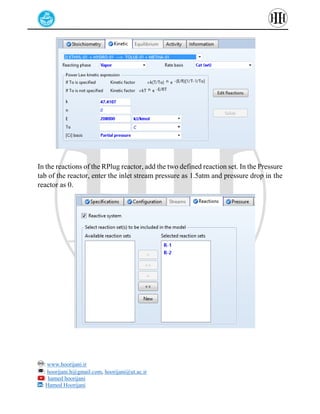
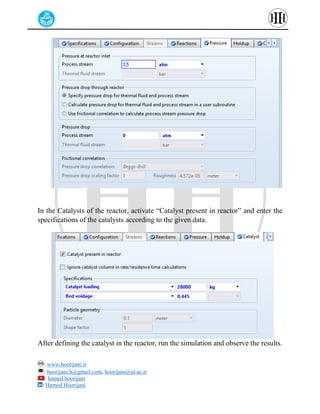
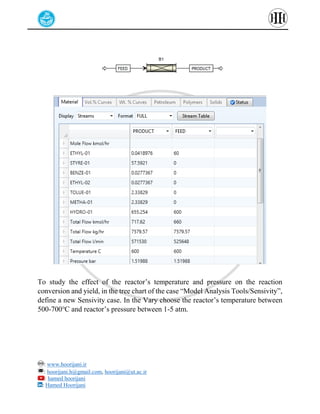
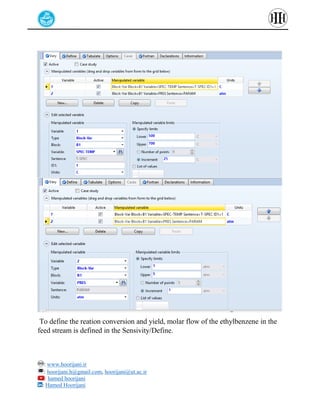
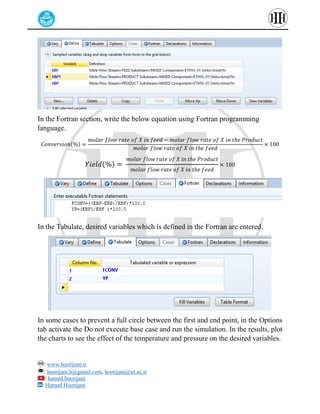
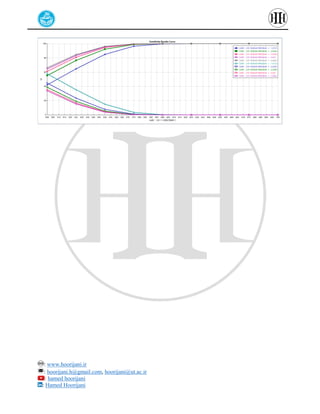
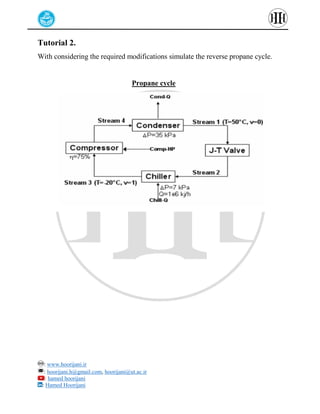
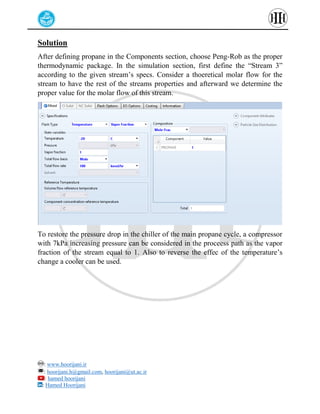
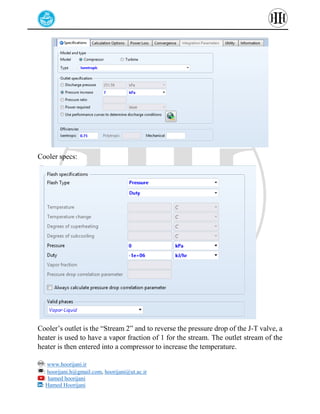
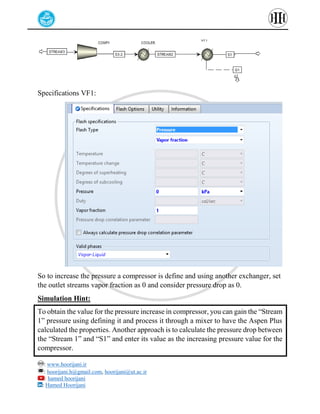
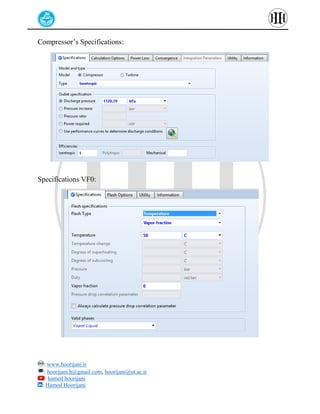
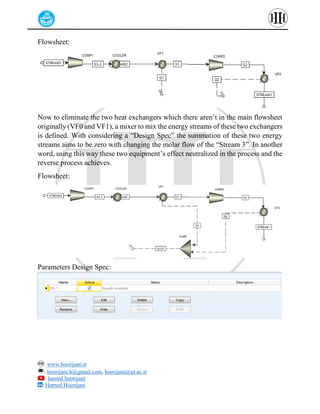
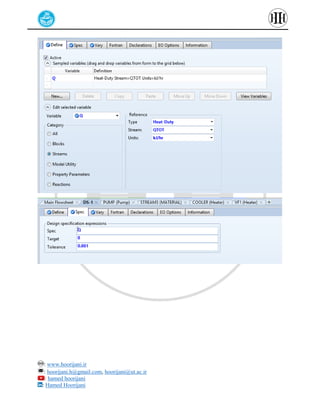
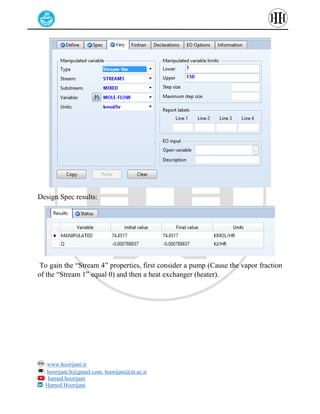
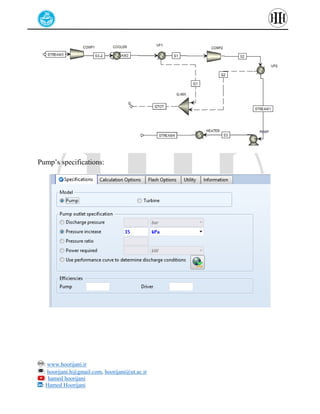
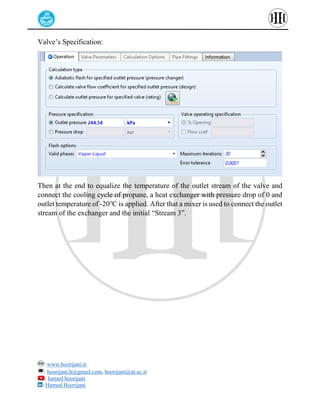
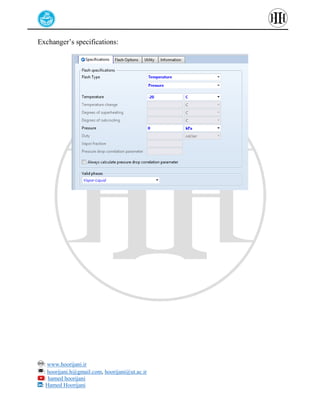
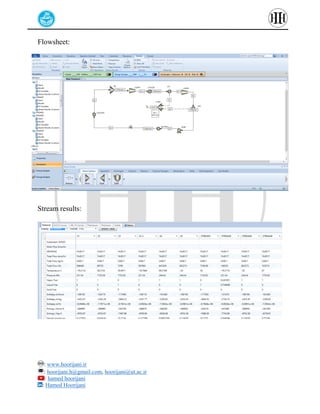
The document provides a detailed tutorial on simulating chemical reactions and processes using Aspen Plus, specifically focusing on the conversion of ethylbenzene to styrene and the reverse propane cycle. It outlines the reaction rates, reactor configurations, thermodynamic packages, and various simulation parameters necessary for analyzing temperature and pressure effects on conversion and yield. Additionally, it includes steps for defining reactions, configuring reactors, and utilizing Fortran for calculations within the Aspen Plus environment.