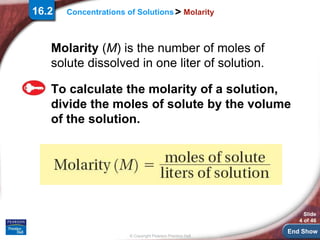
The document is a slide presentation on concentrations of solutions that discusses different ways to calculate and express concentrations, including molarity, percent by volume and mass, and parts per million. It explains how to calculate molarity by dividing moles of solute by liters of solution and the effect of dilution on concentration. It also discusses using volumetric equipment to measure and prepare solutions and working through sample problems calculating concentrations.