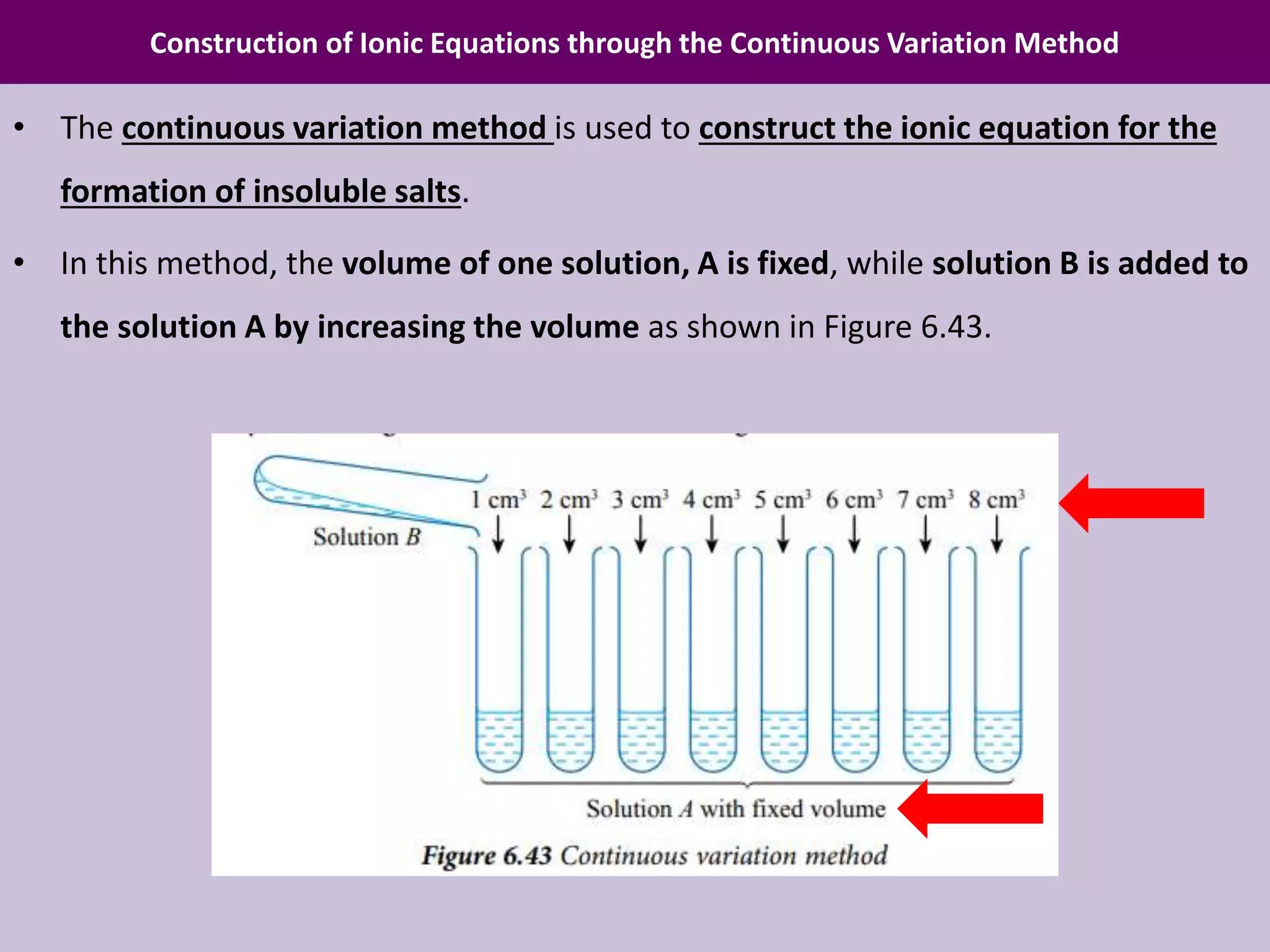
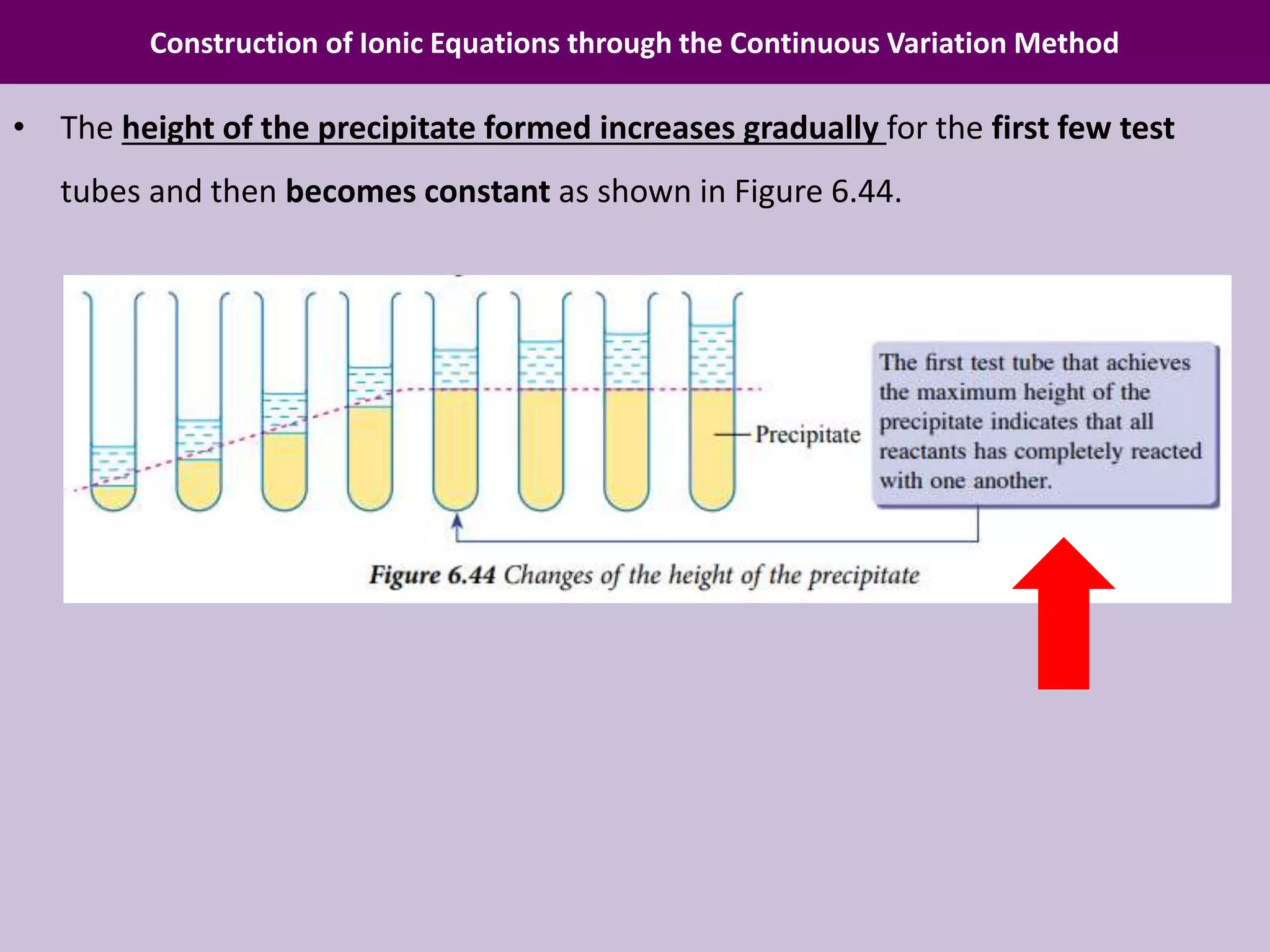
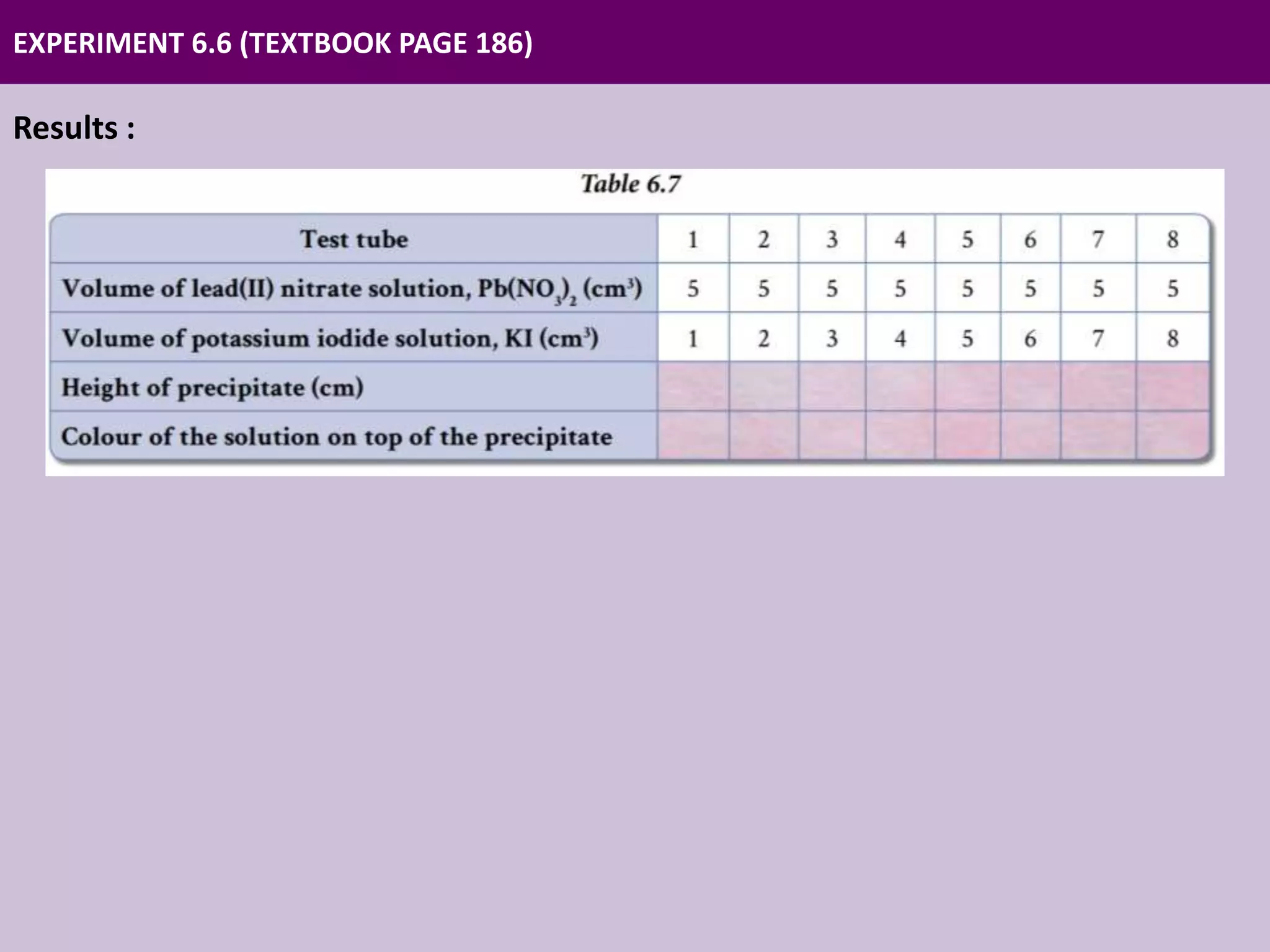
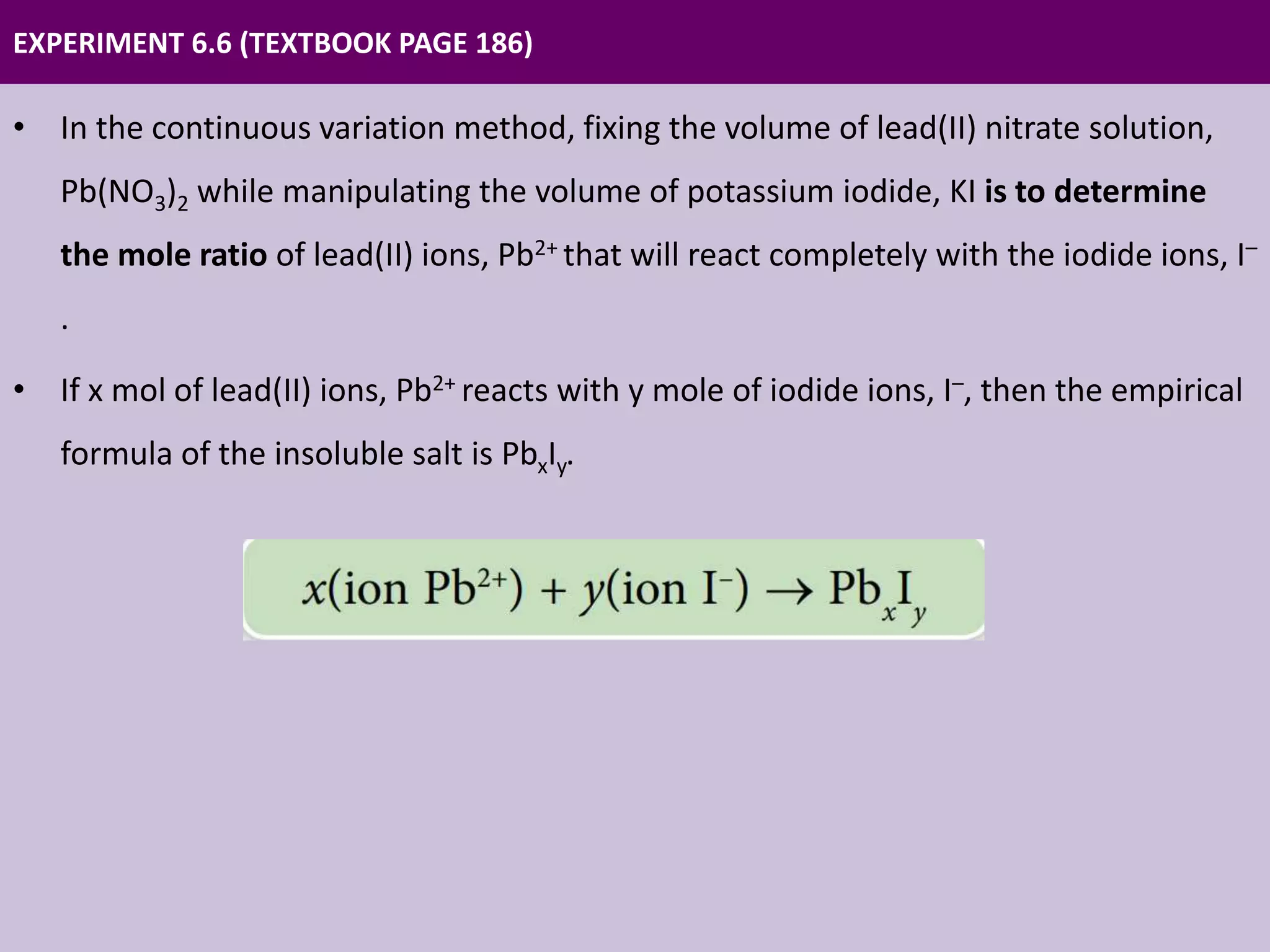
Chapter 6 discusses acids, bases, and salts, outlining their properties, reactions, and applications in daily life. It details an experiment using the continuous variation method to construct ionic equations for the formation of lead(II) iodide, focusing on changes in precipitate height as more potassium iodide is added. The chapter emphasizes careful experimental setup and the importance of manipulating variables to determine chemical relationships.