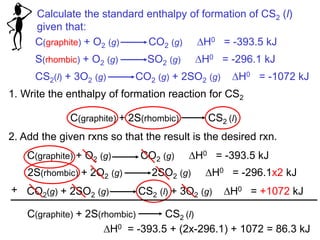
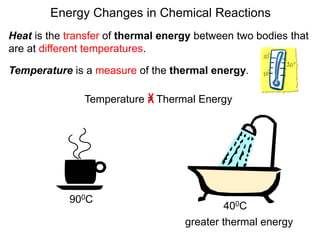
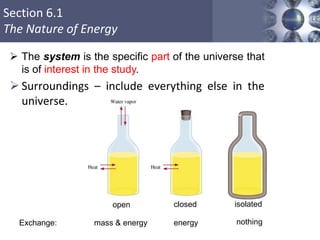
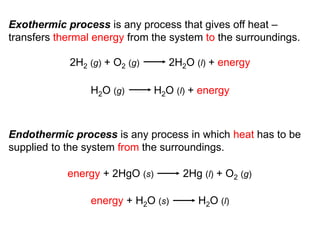
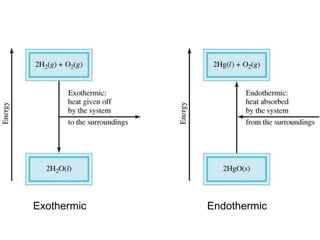
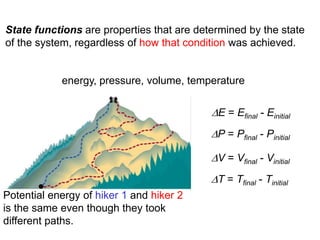
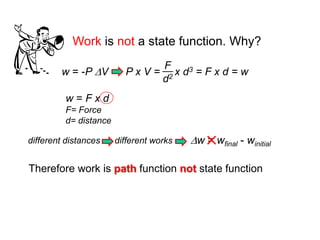
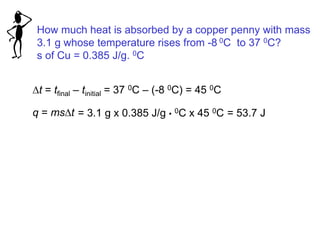
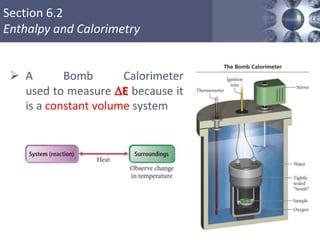
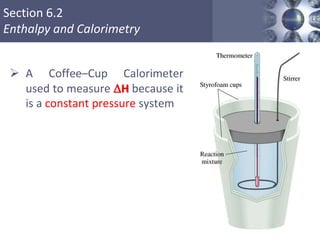
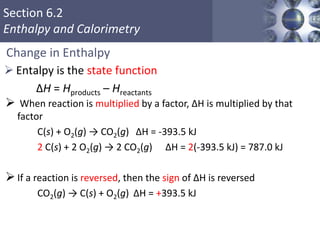
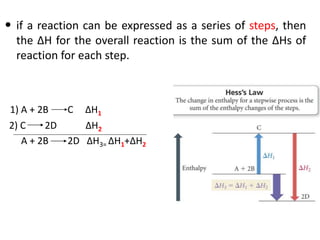
Thermochemistry is the study of heat changes in chemical reactions. Thermal energy is the energy associated with the random motion of atoms and molecules. Enthalpy (H) is a state function that represents the total energy of a system at constant pressure. The standard enthalpy of formation (ΔHf°) is the change in enthalpy that accompanies the formation of one mole of a compound from its elements in their standard states. Hess's law states that the enthalpy change for a reaction is equal to the sum of the enthalpy changes of the steps in any possible pathway between the initial and final states of the reaction.


























![Section 6.4
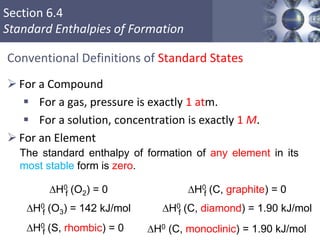
Standard Enthalpies of Formation
Change in enthalpy that accompanies the formation of
one mole of a compound from its elements with all
substances in their standard states.
44
aA + bB cC + dD
DH0 dDH0 (D)
f
cDH0 (C)
f= [ + ] - bDH0 (B)
f
aDH0 (A)
f
[ + ]
DH0
nDH0 (products)
f= S mDH0 (reactants)
f
S-](https://image.slidesharecdn.com/chapter6-thermochemistry1-200610084046/85/Thermochemistry-44-320.jpg)
![Benzene (C6H6) burns in air to produce carbon dioxide and
liquid water. How much heat is released per mole of
benzene combusted? The standard enthalpy of formation
of benzene is 49.04 kJ/mol.
2C6H6 (l) + 15O2 (g) 12CO2 (g) + 6H2O (l)
DH0
rxn nDH0 (products)f= S mDH0 (reactants)fS-
DH0
rxn 6DH0 (H2O)f12DH0 (CO2)f= [ + ] - 2DH0 (C6H6)f[ ]
DH0
rxn = [ 12x–393.5 + 6x–187.6 ] – [ 2x49.04 ] = -5946 kJ
-5946 kJ
2 mol
= - 2973 kJ/mol C6H6
6.6](https://image.slidesharecdn.com/chapter6-thermochemistry1-200610084046/85/Thermochemistry-47-320.jpg)