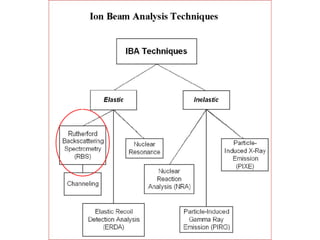
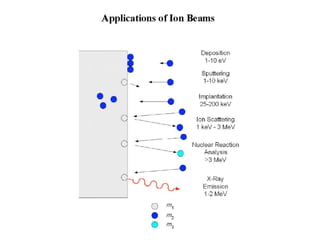
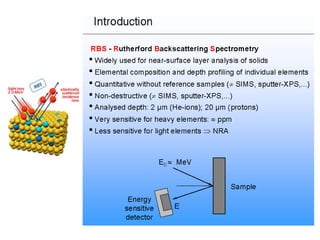
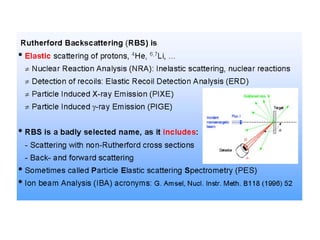
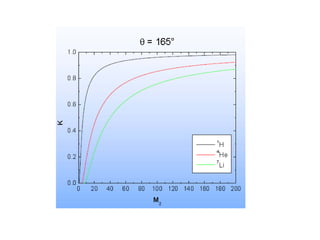
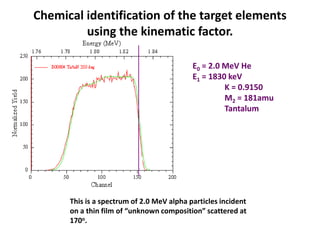
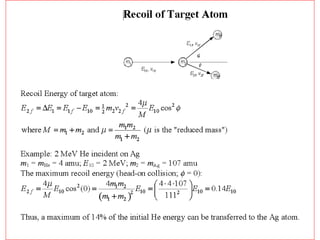
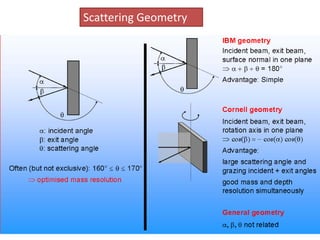
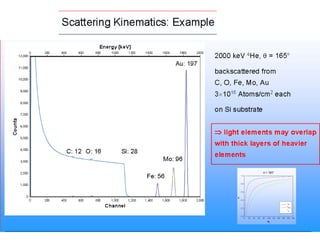
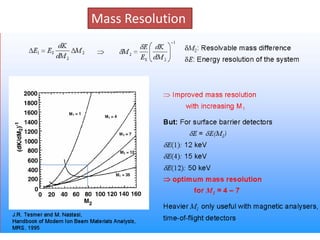
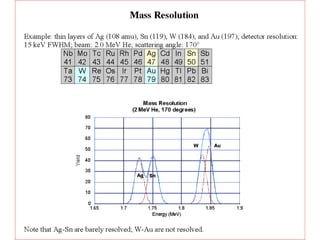
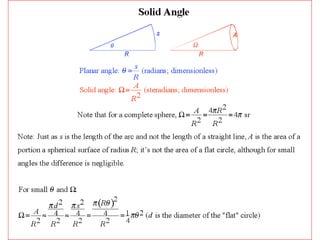
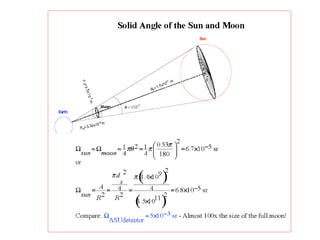
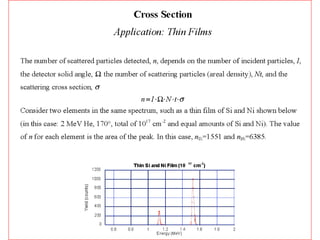
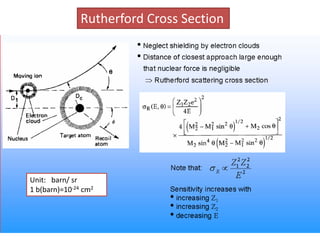
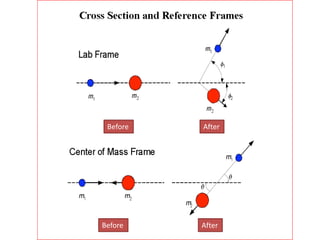
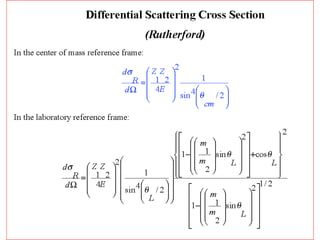
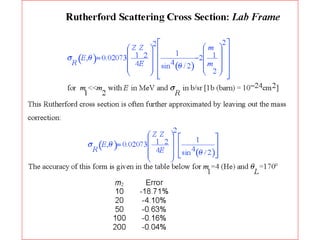
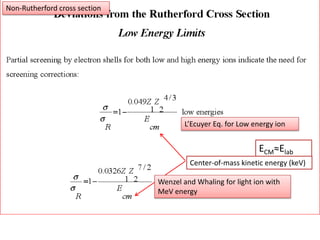
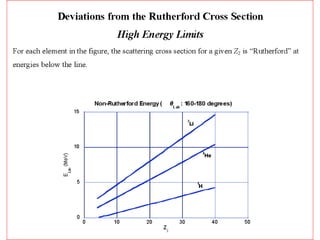
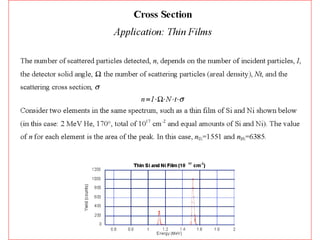
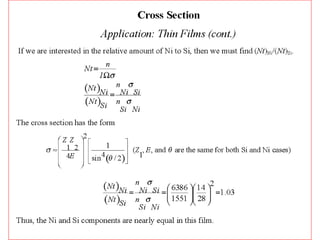
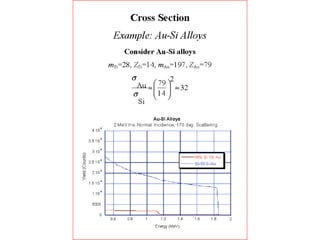
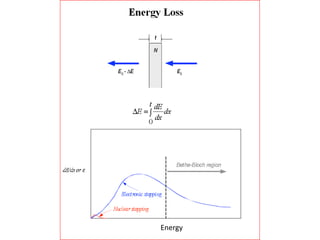
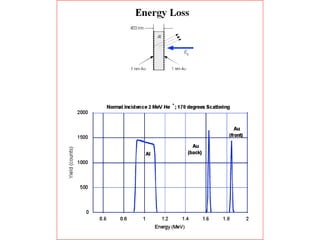
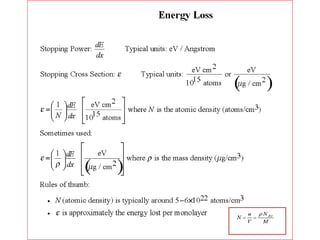
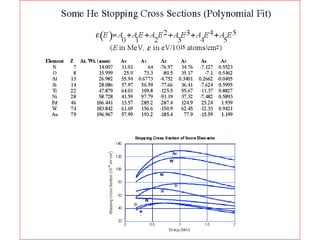
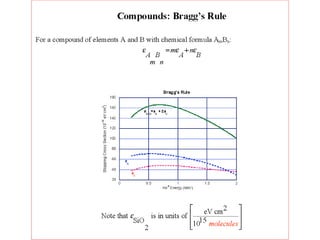
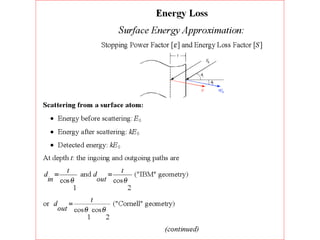
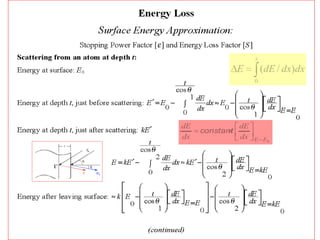
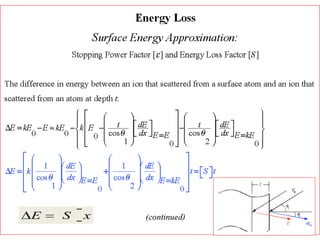
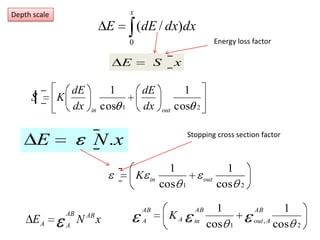
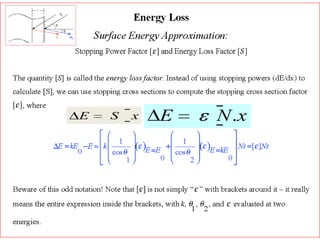
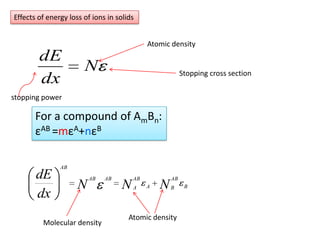
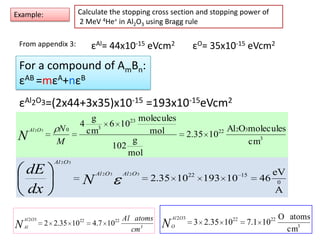
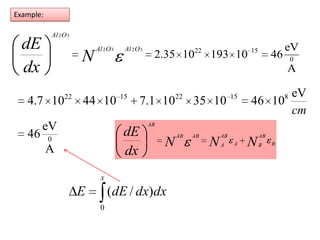
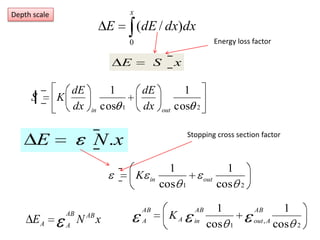
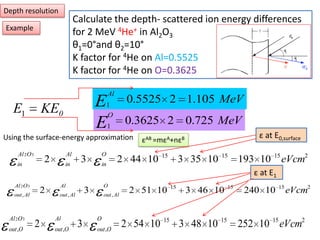
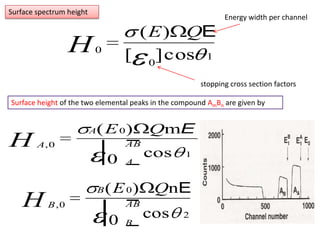
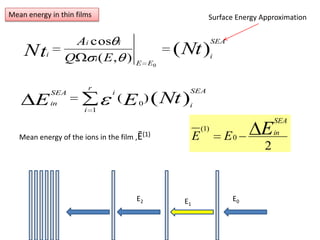
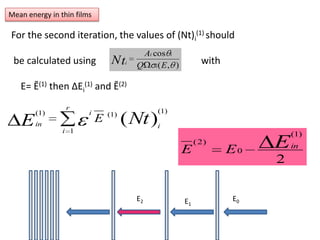
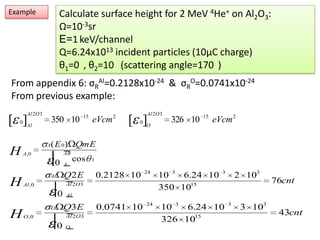
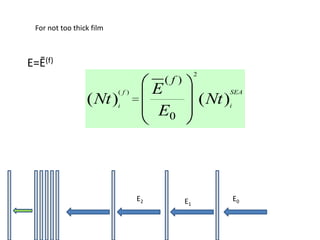
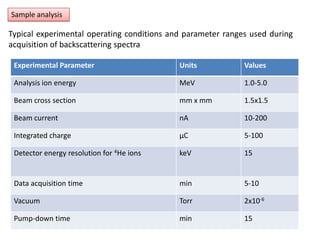
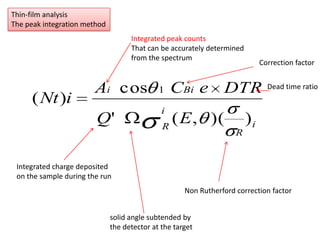
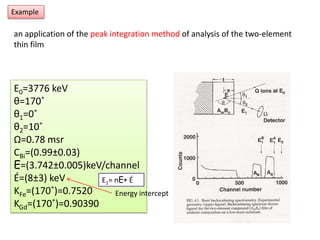
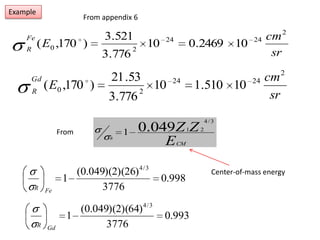
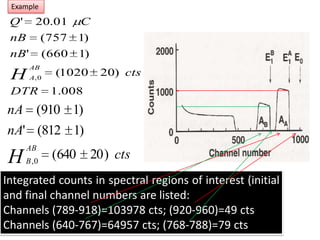
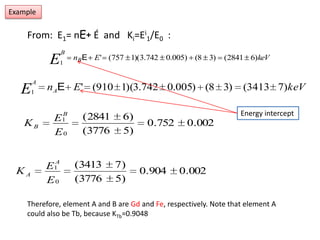
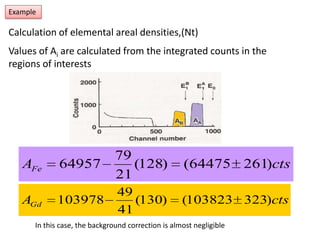
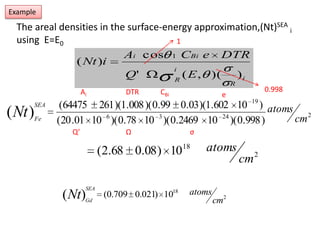
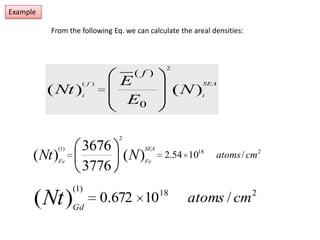
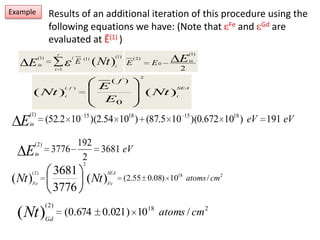
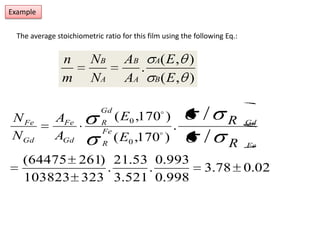
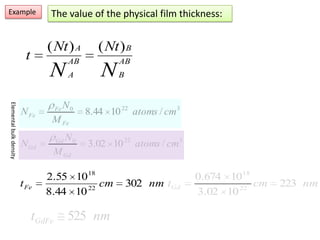
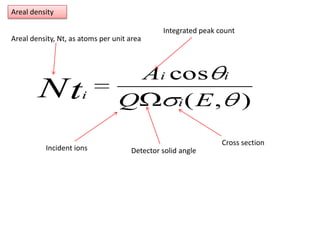
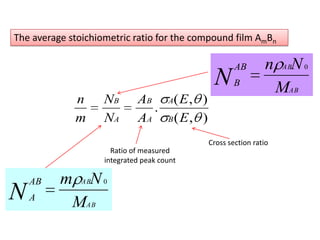
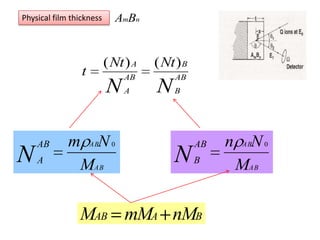
This document discusses backscattering spectrometry, which uses elastic scattering of ions to determine the elemental composition of materials. It describes how Rutherford scattering can be used for low energy particles, while higher energies require solving the Schrodinger equation. Examples are given of using kinematic factors to identify elements in a spectrum and calculating stopping power and cross sections. The document outlines approaches for thin film analysis using peak integration and mean energy calculations to determine areal densities and stoichiometry.