This chapter discusses the key concepts and gas laws relating to gases:
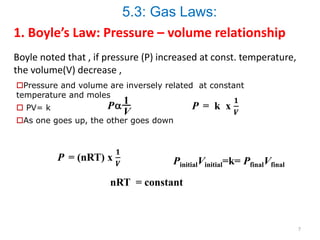
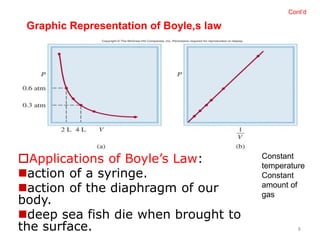
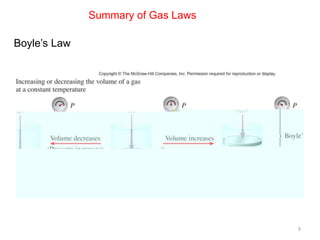
1) Boyle's law states that at a constant temperature, the pressure and volume of a gas are inversely proportional.
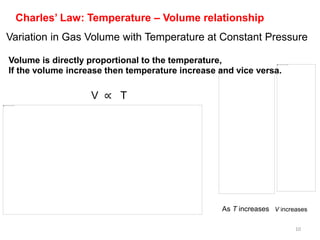
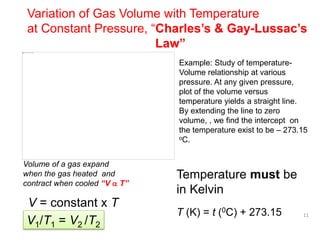
2) Charles' law describes the direct relationship between volume and temperature of a gas at constant pressure.
3) Avogadro's law relates the volume and amount of gas present at constant pressure and temperature.
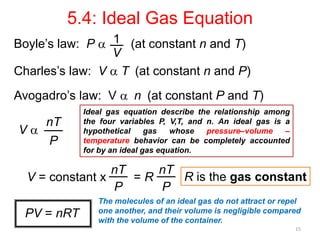
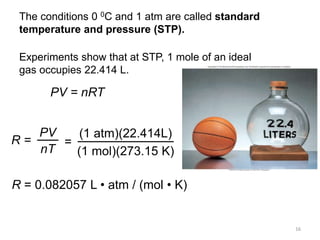
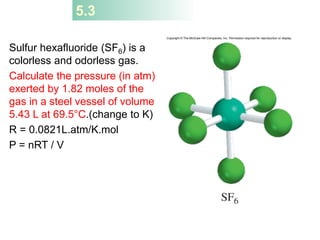
4) The ideal gas law combines these relationships between pressure, volume, temperature, and amount of gas.
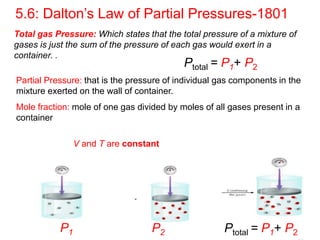
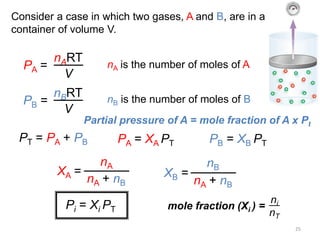
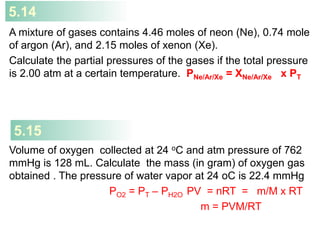
5) Dalton's law of partial pressures describes how the total pressure of a gas mixture is equal to the sum of the individual gas partial pressures.





![13
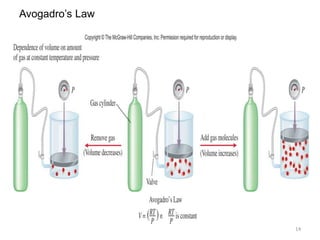
Avogadro’s Law: [Volume – Amount relationship]
Avogadro’s complimented the studies of Boyle, Charles, and Gay-
Lussac.: He published a hypothesis stating that at the same temperature
and pressure , equal volume of all gases contain the same number of
molecules (or atoms).
V a number of moles (n)
V = k x n V1 / n1 = V2 / n2
Constant temperature &Constant pressure
n represent the no. of moles and k is the
proportionality constant.
3H2 (g) + N2 (g) 2NH3 (g).
3mol 1 mol 2 mol
3H2 (g) + N2 (g) 2NH3 (g).
3 volume 1volume 2 volume](https://image.slidesharecdn.com/chapter5-gasesreduced1-160930162747/85/Chapter-5-gases-reduced1-13-320.jpg)
![20
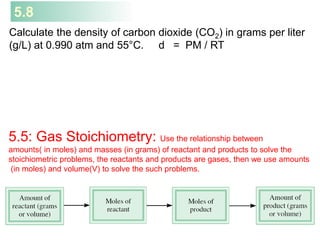
Density (d) Calculations [n /V = P / RT], n = m/MM
m
MV
P
RT=
m is the mass of the gas in g
M is the molar mass of the gas
Molar Mass (M ) of a Gaseous Substance
dRT
P
M = d is the density of the gas in g/L
d = m
V
=
PM
RT](https://image.slidesharecdn.com/chapter5-gasesreduced1-160930162747/85/Chapter-5-gases-reduced1-20-320.jpg)