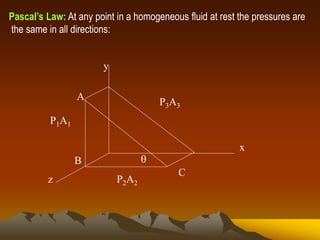
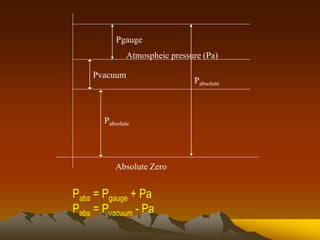
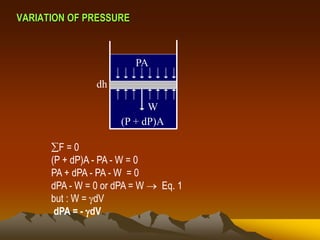
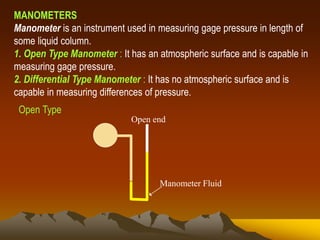
Here are the key steps to solve this problem:
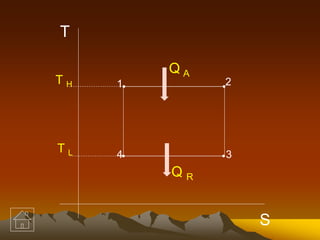
1. Given: TH = 817°C = 817 + 273 = 1090 K
TL = 25°C = 25 + 273 = 298 K
QR = 25 kW
2. Use the Carnot efficiency equation:
η = (TH - TL)/TH = (1090 - 298)/1090 = 0.726
3. Set up an equation for the heat input using the efficiency and heat rejected:
QA = QR/(1-η) = 25000/(1-0.726) = 87500 kW
Therefore, the heat input (QA) required is 87500 kW.