The document describes various thermodynamic processes including:
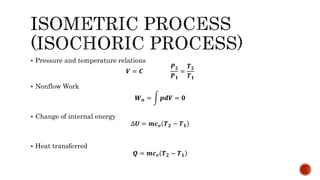
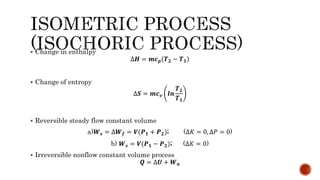
1) Isometric processes which occur at constant volume with changes in temperature and pressure.
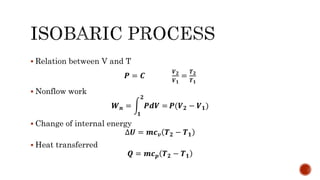
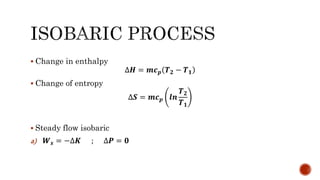
2) Isobaric processes which occur at constant pressure with changes in volume and temperature.
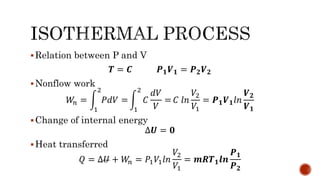
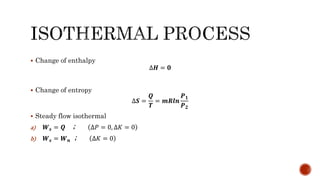
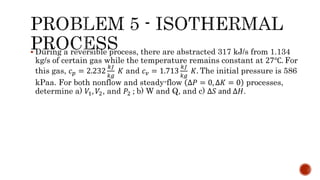
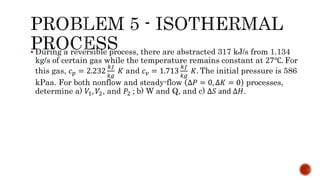
3) Isothermal processes which occur at constant temperature with changes in pressure and volume.
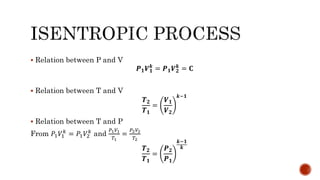
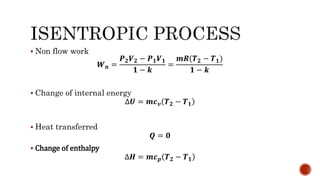
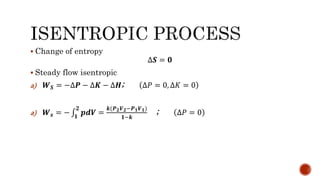
4) Isentropic processes which are reversible adiabatic processes with no heat transfer and constant entropy.
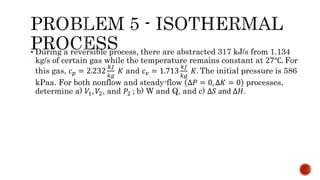
Several examples are provided to calculate temperature, pressure, volume, work, heat, internal energy and entropy changes during these different thermodynamic processes.