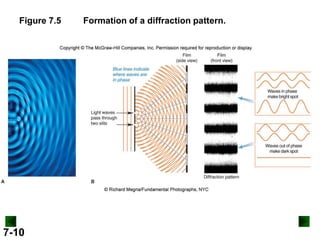
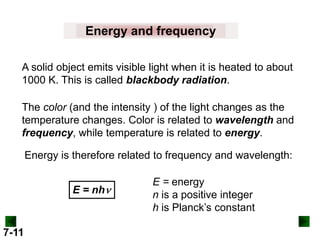
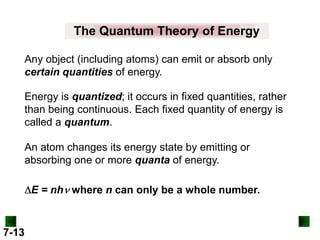
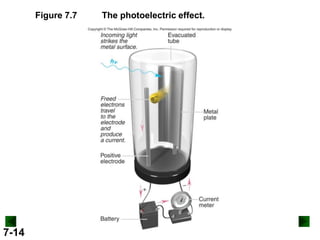
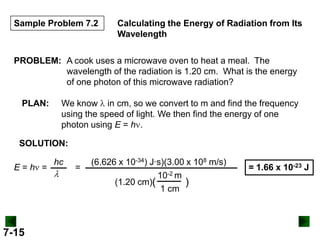
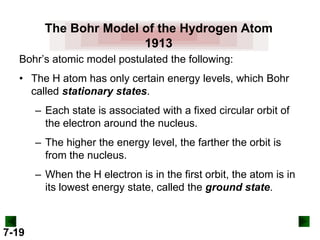
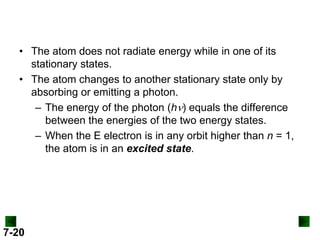
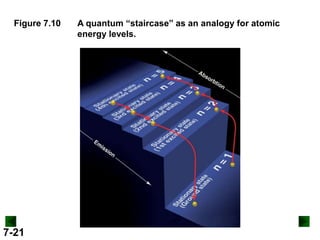
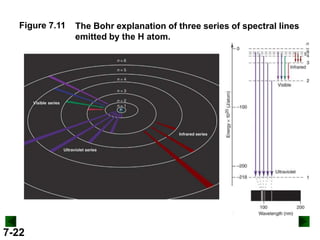
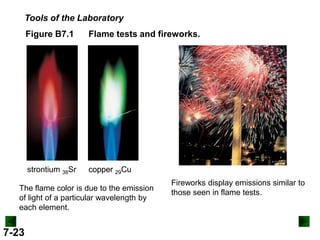
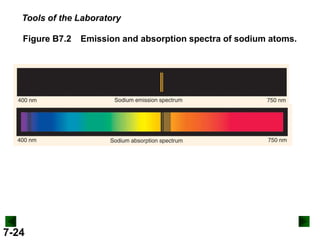
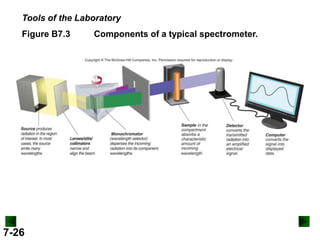
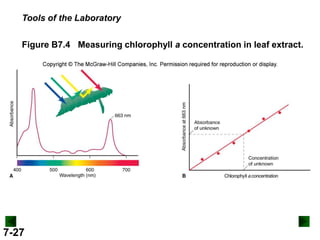
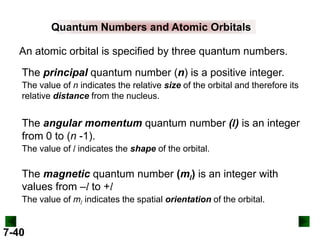
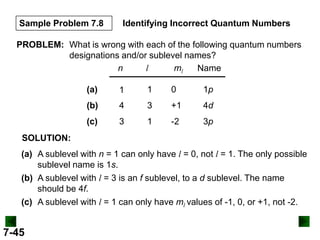
This document provides information about quantum theory and atomic structure:
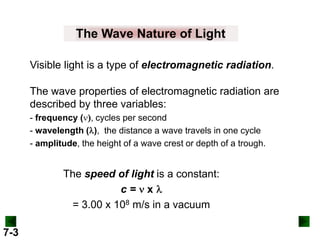
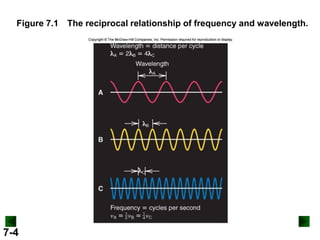
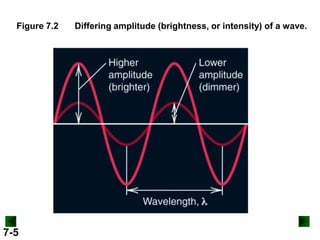
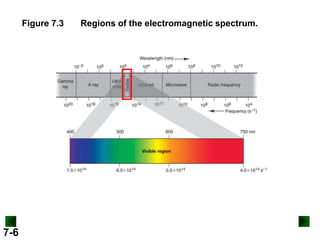
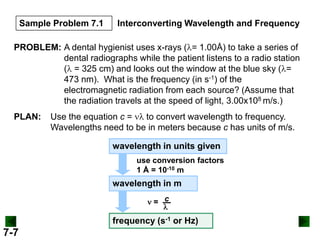
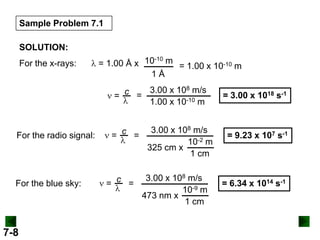
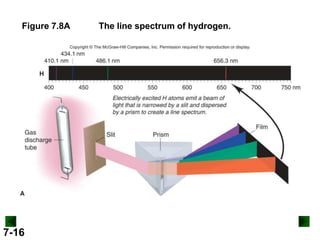
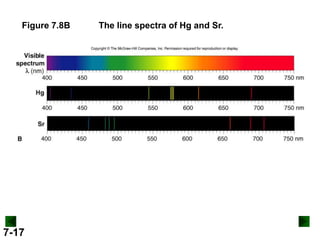
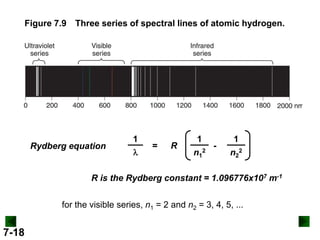
- It introduces the wave nature of light and electromagnetic radiation, including frequency, wavelength, and speed of light.
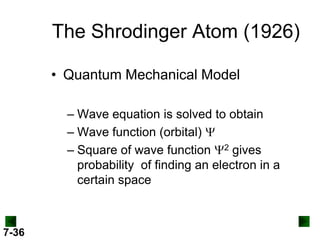
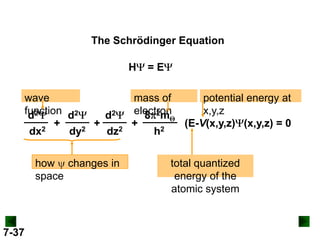
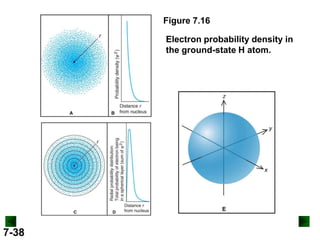
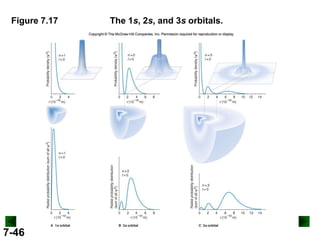
- Models of the atom are discussed, from the Bohr model to the quantum mechanical model using the Schrodinger wave equation.
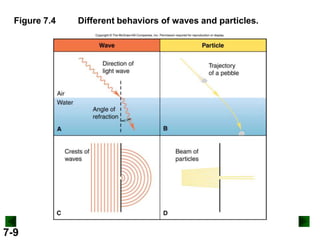
- Key concepts in quantum theory are explained, such as quantization of energy, photons, wave-particle duality, and the Heisenberg uncertainty principle.
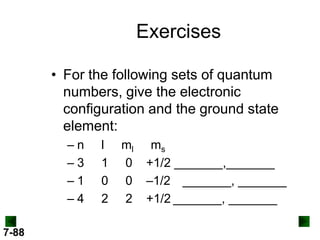
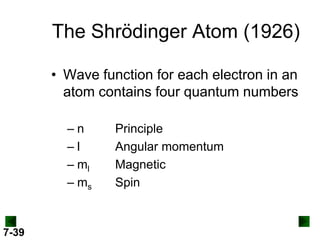
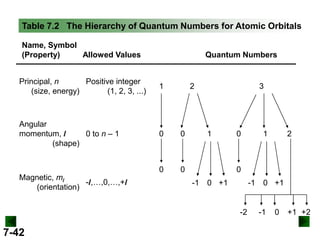
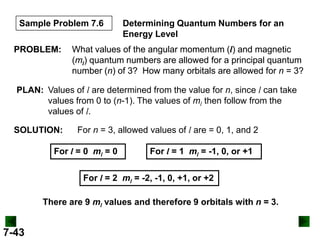
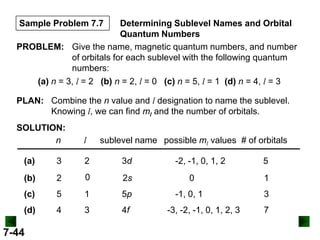
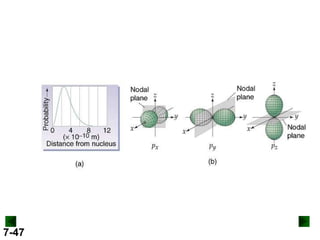
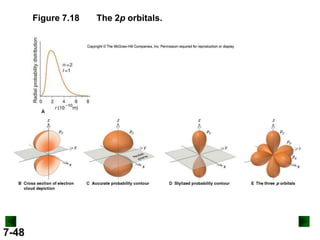
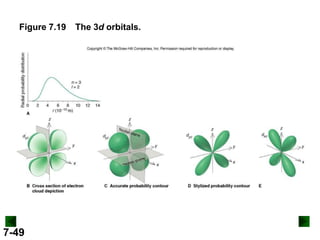
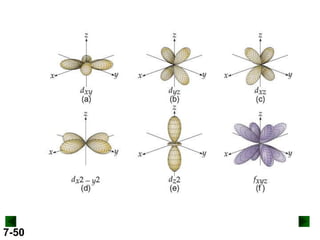
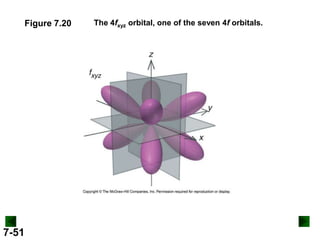
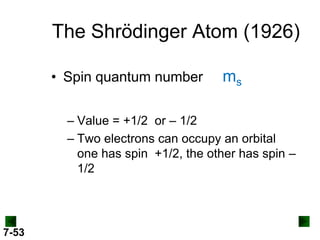
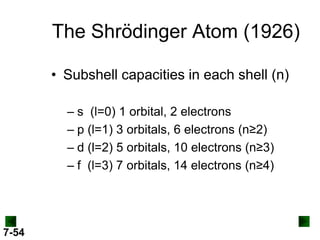
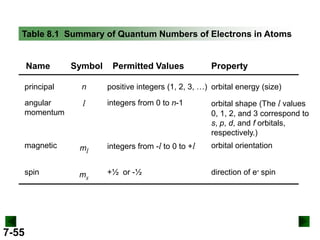
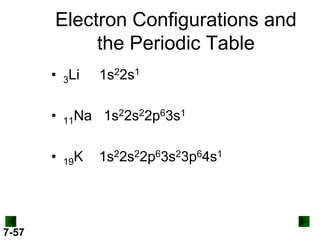
- Atomic orbitals are described using quantum numbers such as principal, angular momentum, and magnetic, and how these relate to electron configuration.












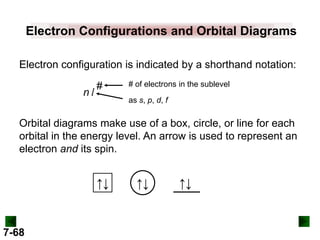
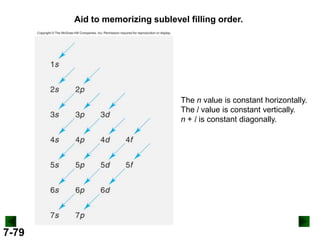
![Partial Orbital Diagrams and
Condensed Configurations
A partial orbital diagram shows only the highest energy
sublevels being filled.
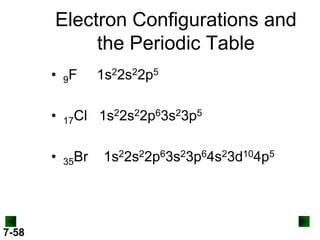
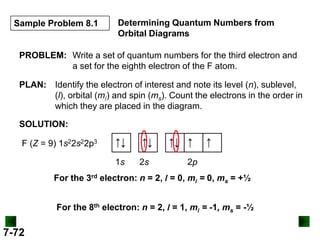
Al (Z = 13) 1s22s22p63s23p1
↑↓
3s
↑
3p
A condensed electron configuration has the element
symbol of the previous noble gas in square brackets.
Al has the condensed configuration [Ne]3s23p1
7-73](https://image.slidesharecdn.com/new-chm-151-unit-4-power-points-140227172225-phpapp02/85/New-chm-151_unit_4_power_points-73-320.jpg)


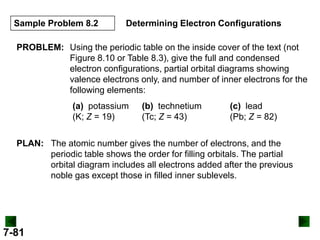
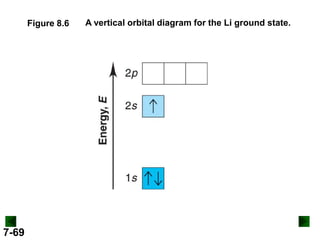
![Sample Problem 8.2
SOLUTION:
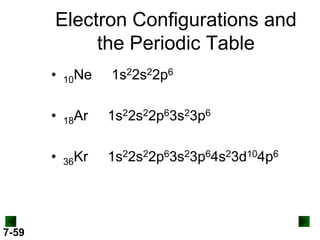
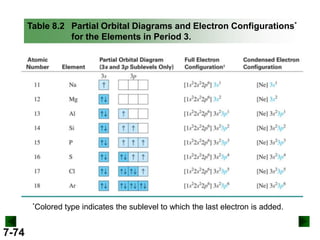
(a) For K (Z = 19)
full configuration
1s22s22p63s23p64s1
condensed configuration
[Ar] 4s1
partial orbital diagram
↑
4s
3d
There are 18 inner electrons.
7-82
4p](https://image.slidesharecdn.com/new-chm-151-unit-4-power-points-140227172225-phpapp02/85/New-chm-151_unit_4_power_points-82-320.jpg)
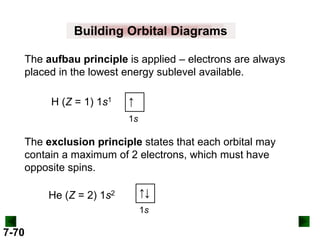
![Sample Problem 8.2
SOLUTION:
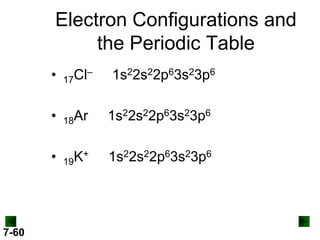
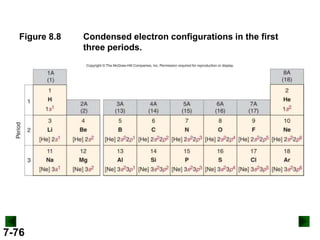
(b) For Tc (Z = 43)
full configuration
1s22s22p63s23p64s23d104p65s24d5
condensed configuration
[Kr]5s24d5
partial orbital diagram
↑↓
5s
↑
↑
↑
↑
4d
There are 36 inner electrons.
7-83
↑
5p](https://image.slidesharecdn.com/new-chm-151-unit-4-power-points-140227172225-phpapp02/85/New-chm-151_unit_4_power_points-83-320.jpg)
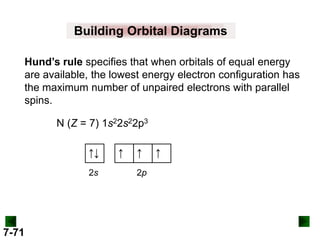
![Sample Problem 8.2
SOLUTION:
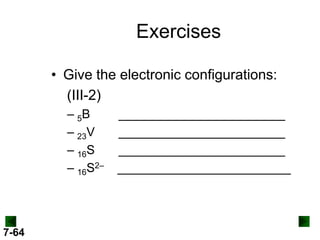
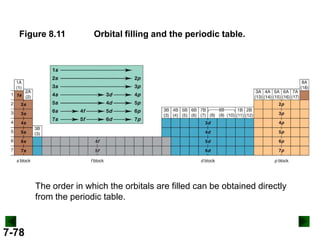
(c) For Pb (Z = 82)
full configuration
1s22s22p63s23p64s23d104p65s24d105p66s24f145d106p2
[Xe] 6s24f145d106p2
condensed configuration
partial orbital diagram
↑↓
6s
↑
↑
6p
There are 78 inner electrons.
7-84](https://image.slidesharecdn.com/new-chm-151-unit-4-power-points-140227172225-phpapp02/85/New-chm-151_unit_4_power_points-84-320.jpg)