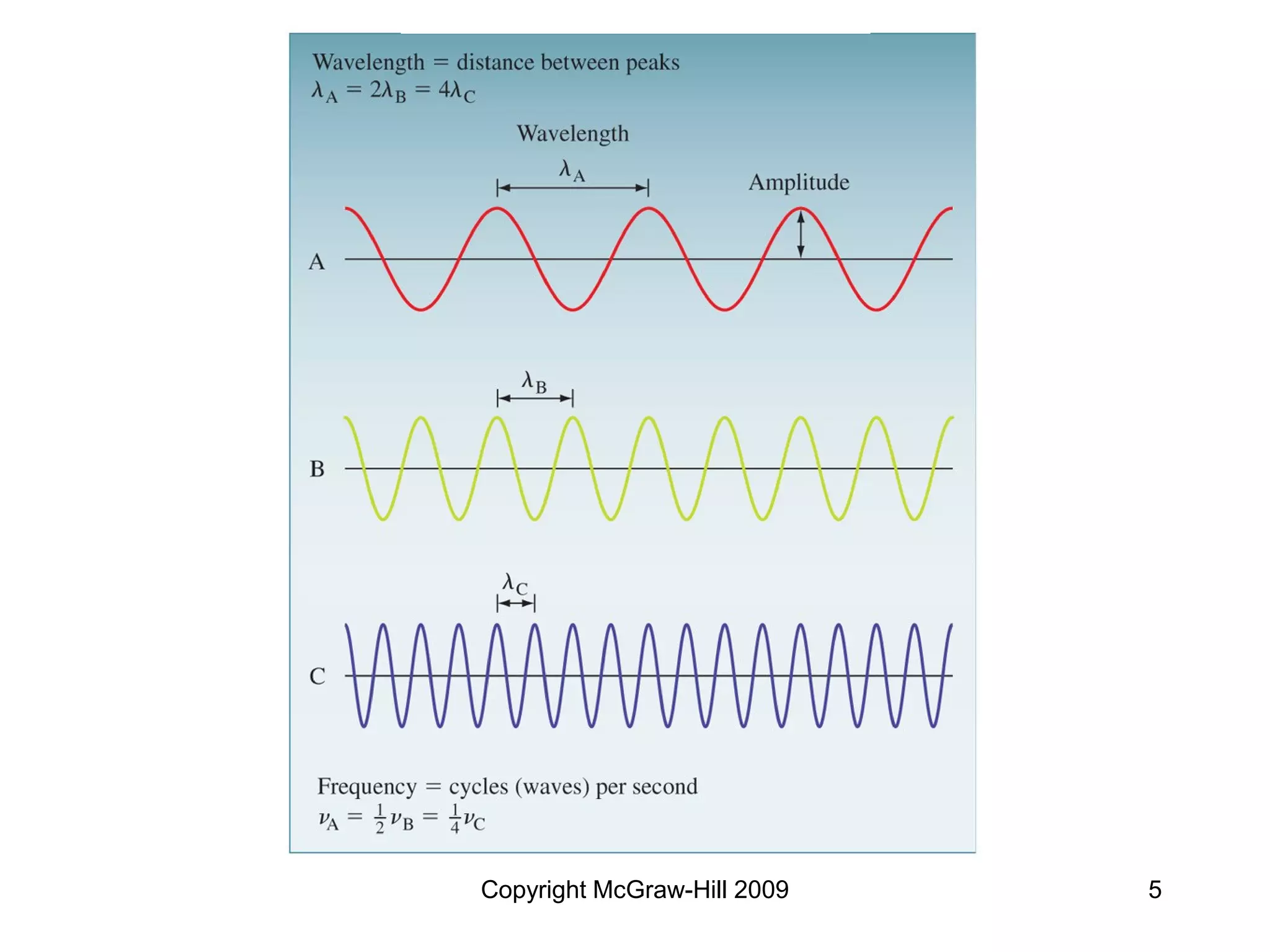
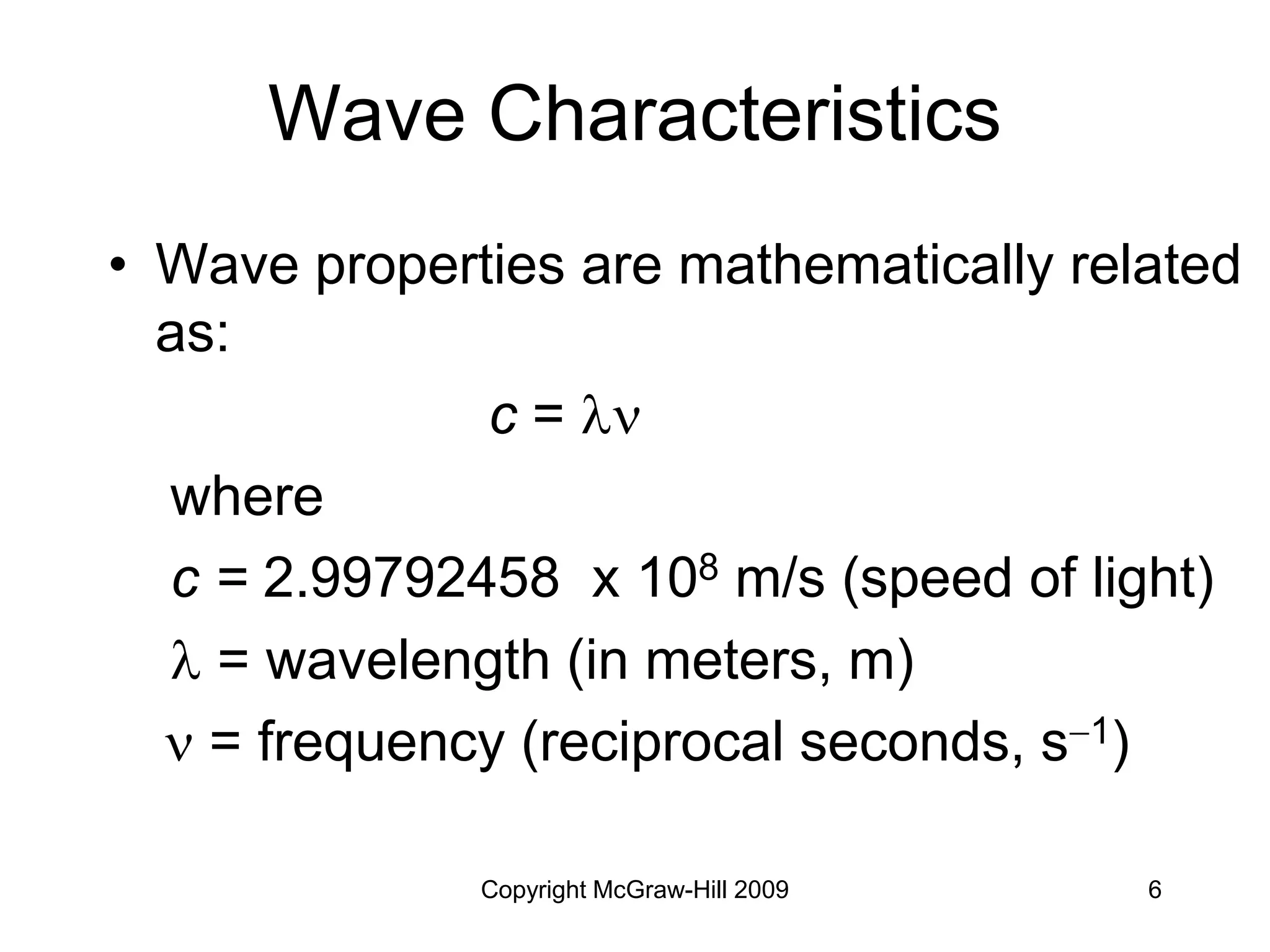
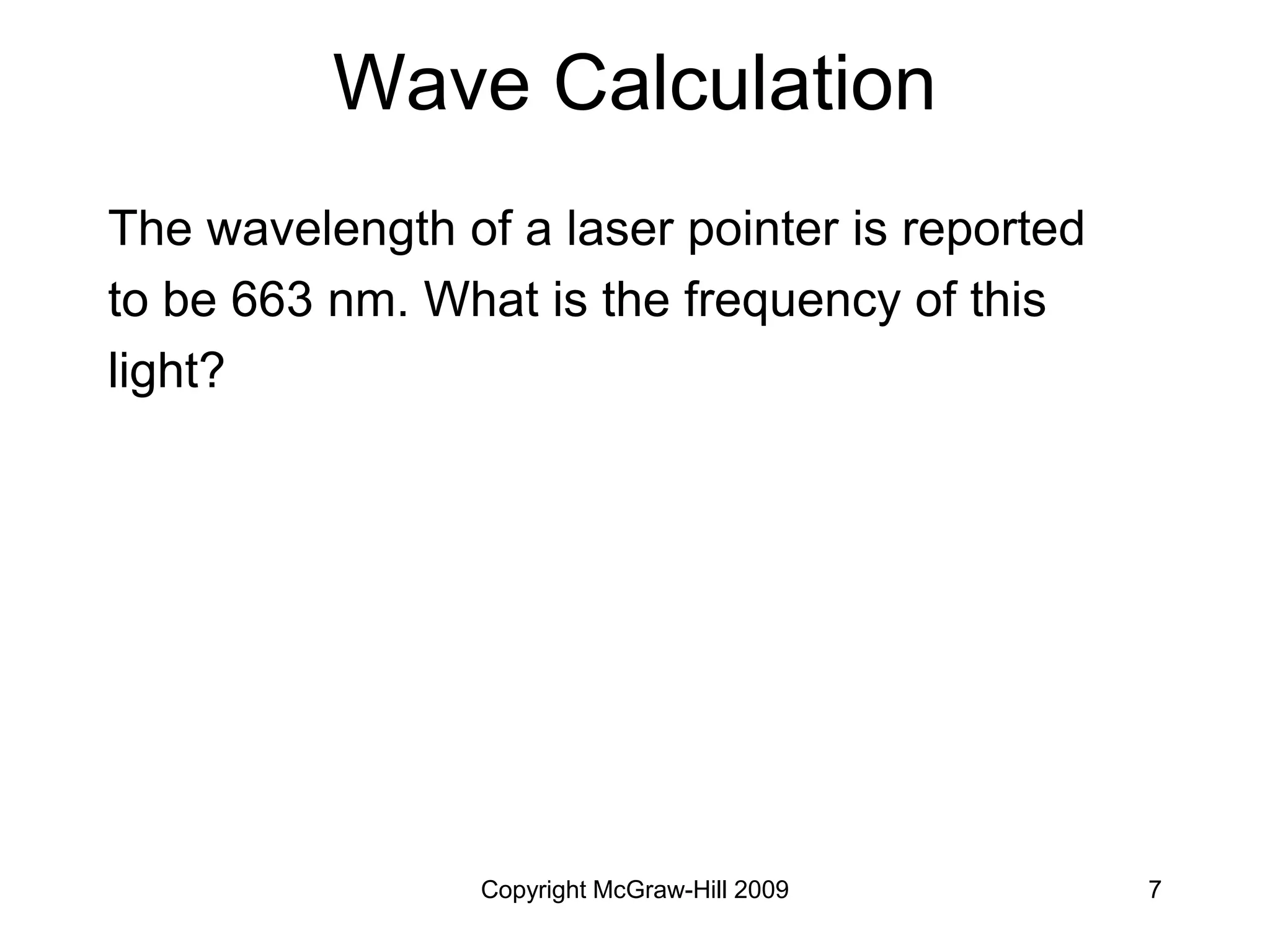
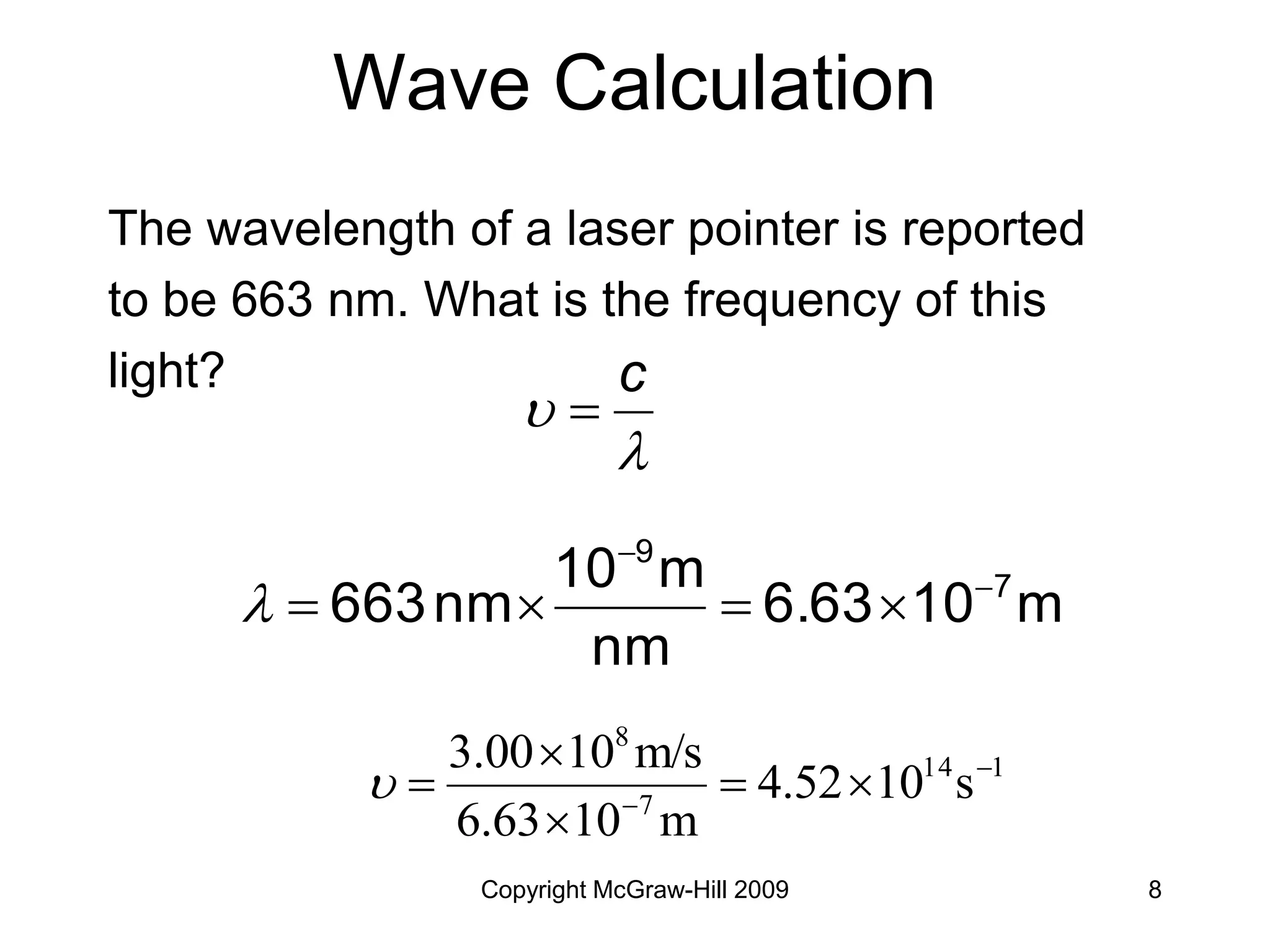
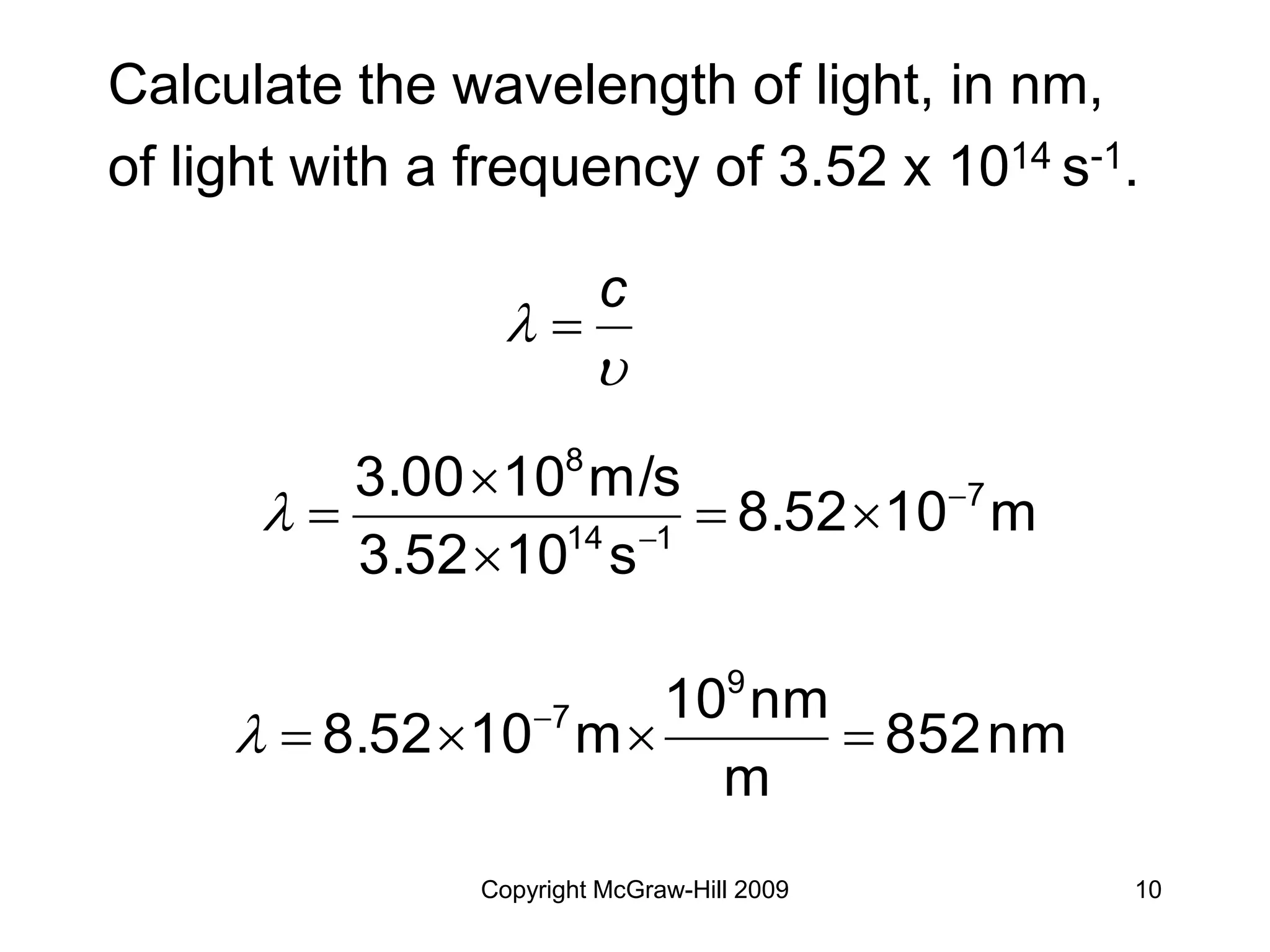
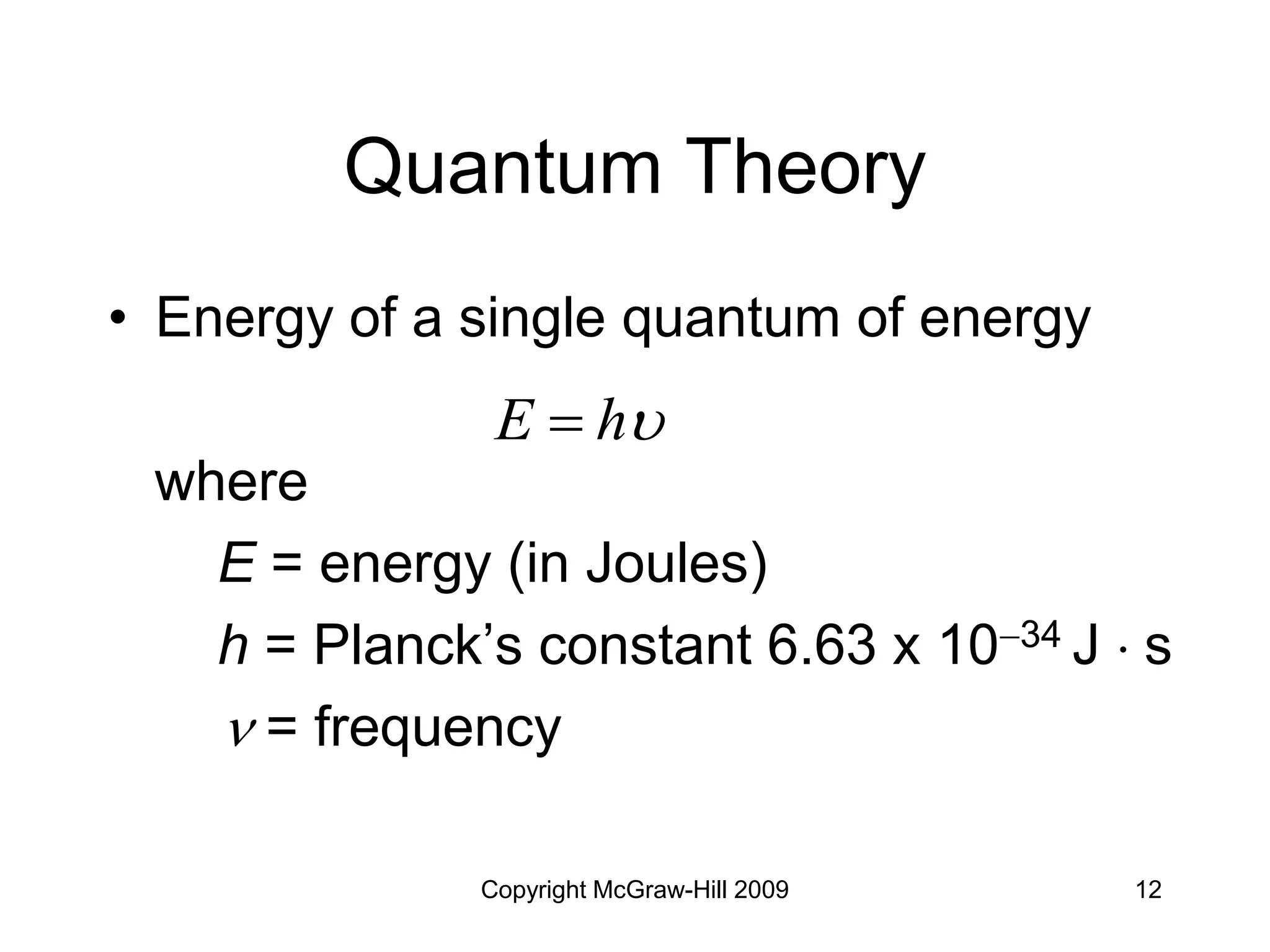
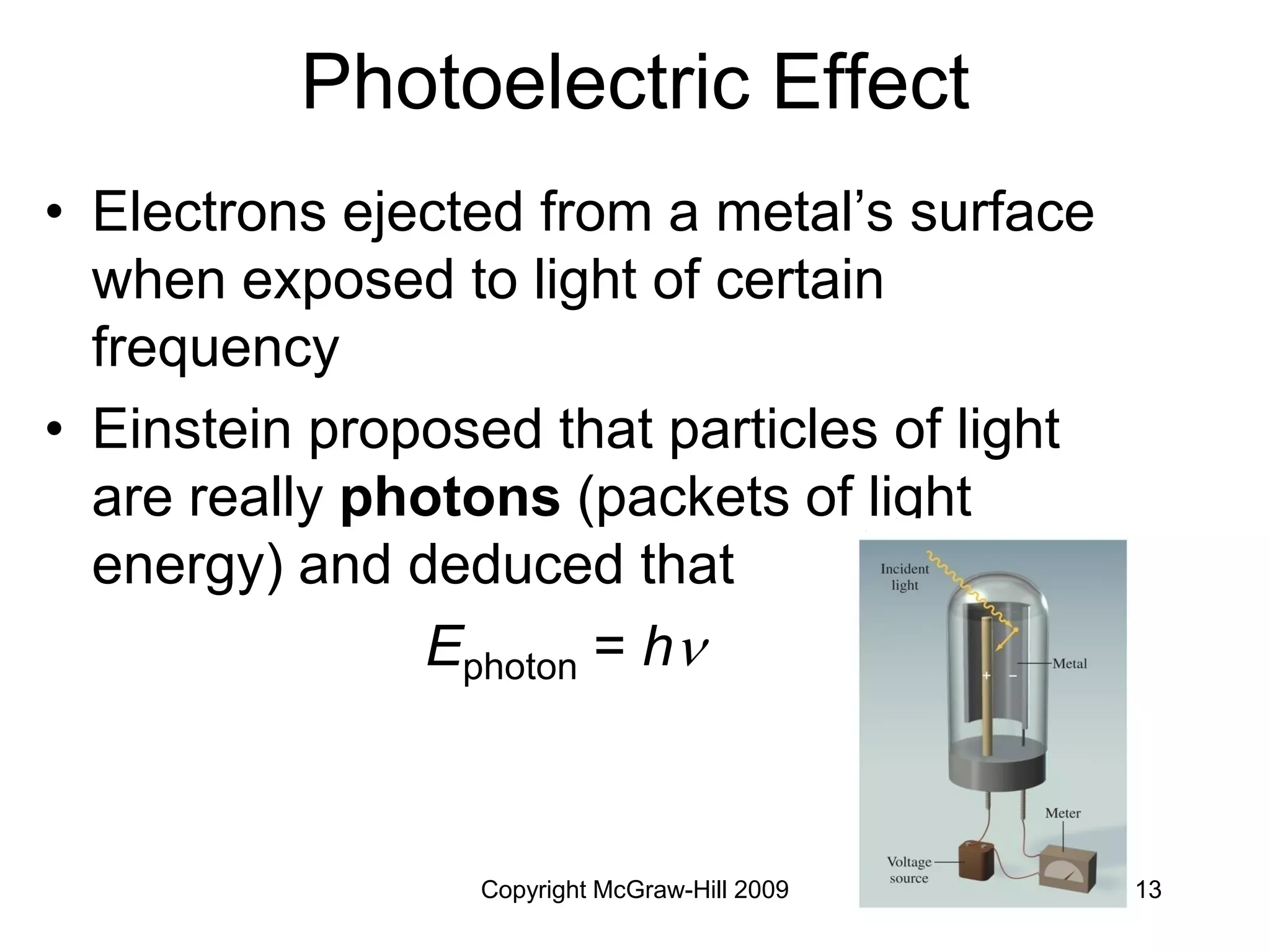
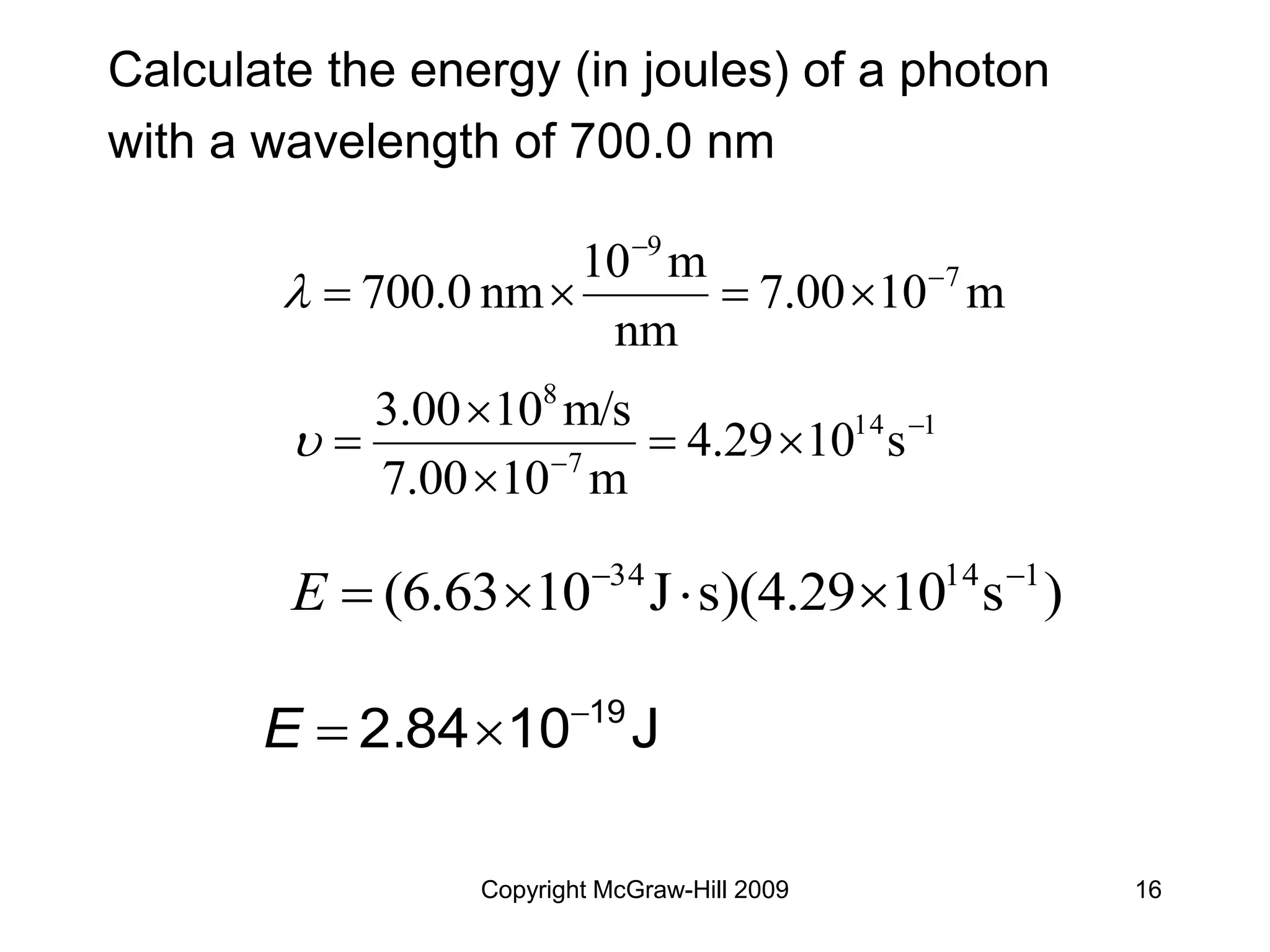
This document provides an overview of quantum theory and the electronic structure of atoms. It begins by discussing the nature of light and electromagnetic radiation. It then covers Max Planck's quantum theory, Albert Einstein's explanation of the photoelectric effect, and Niels Bohr's model of the hydrogen atom. The document also discusses Louis de Broglie's idea of wave-particle duality and the development of quantum mechanics by Werner Heisenberg and Erwin Schrödinger. It introduces the concept of atomic orbitals and quantum numbers that describe the structure of atoms. The document concludes with a discussion of electron configuration and the distribution of electrons in different atomic orbitals.


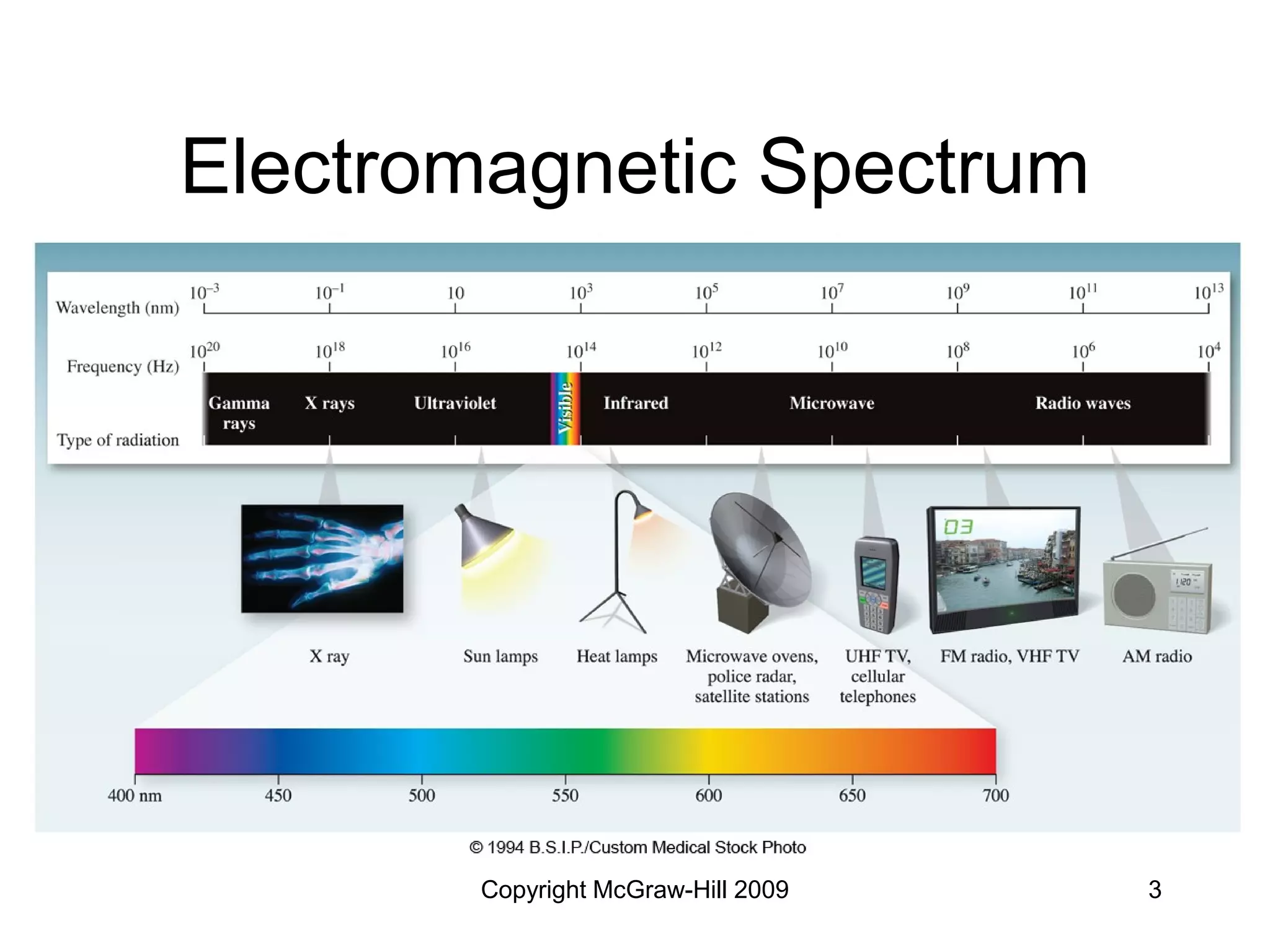
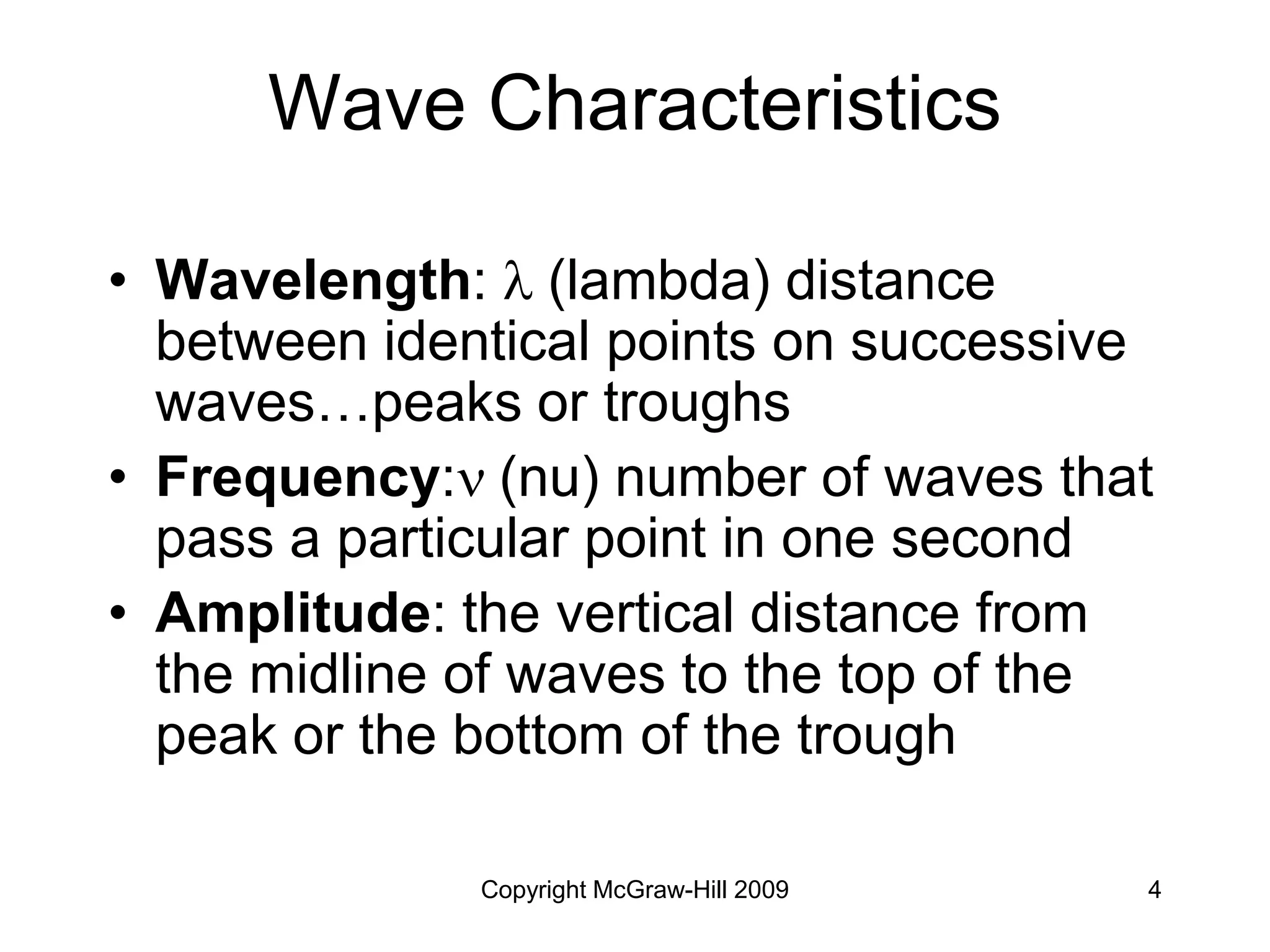















![Copyright McGraw-Hill 2009 64
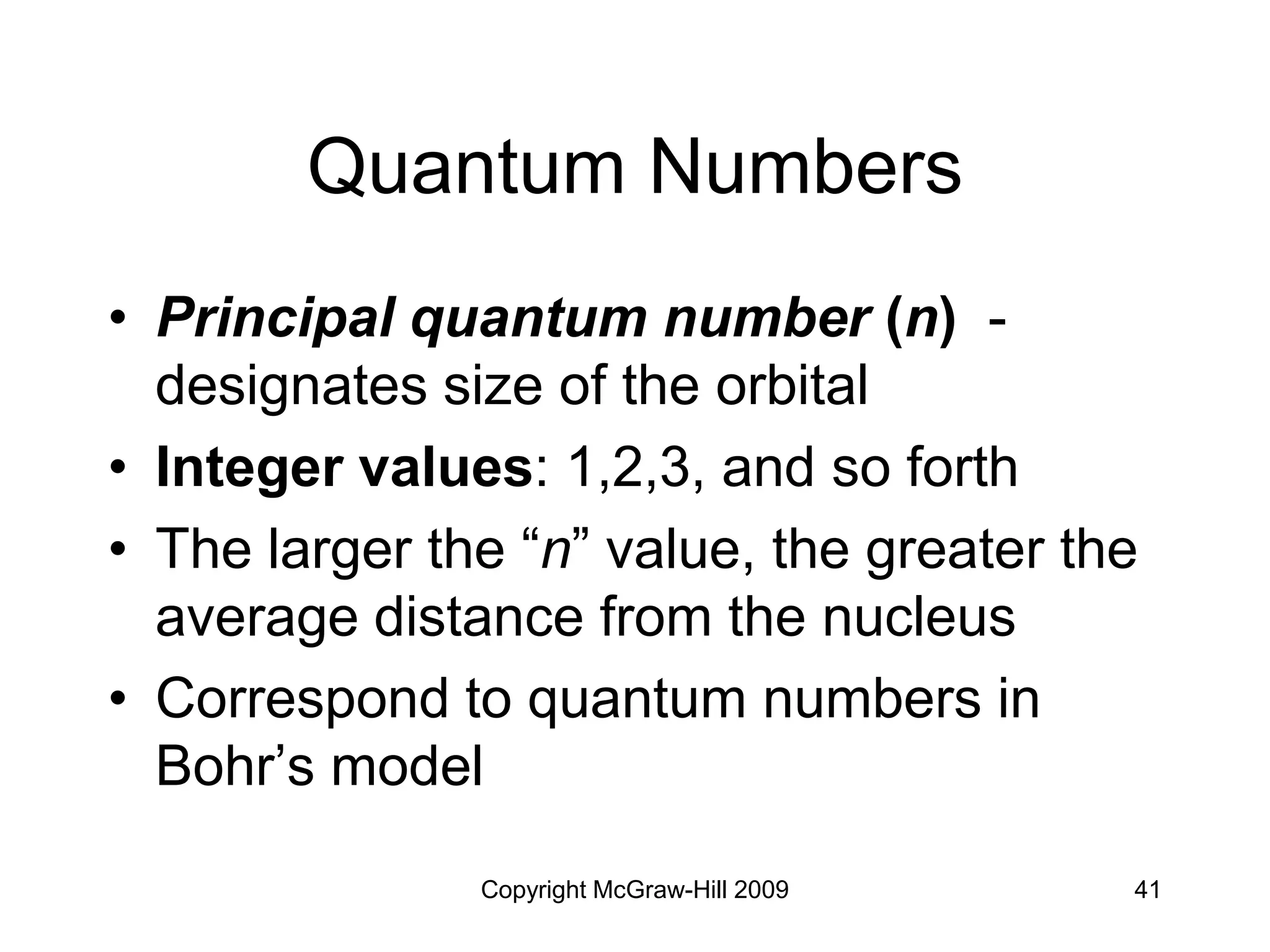
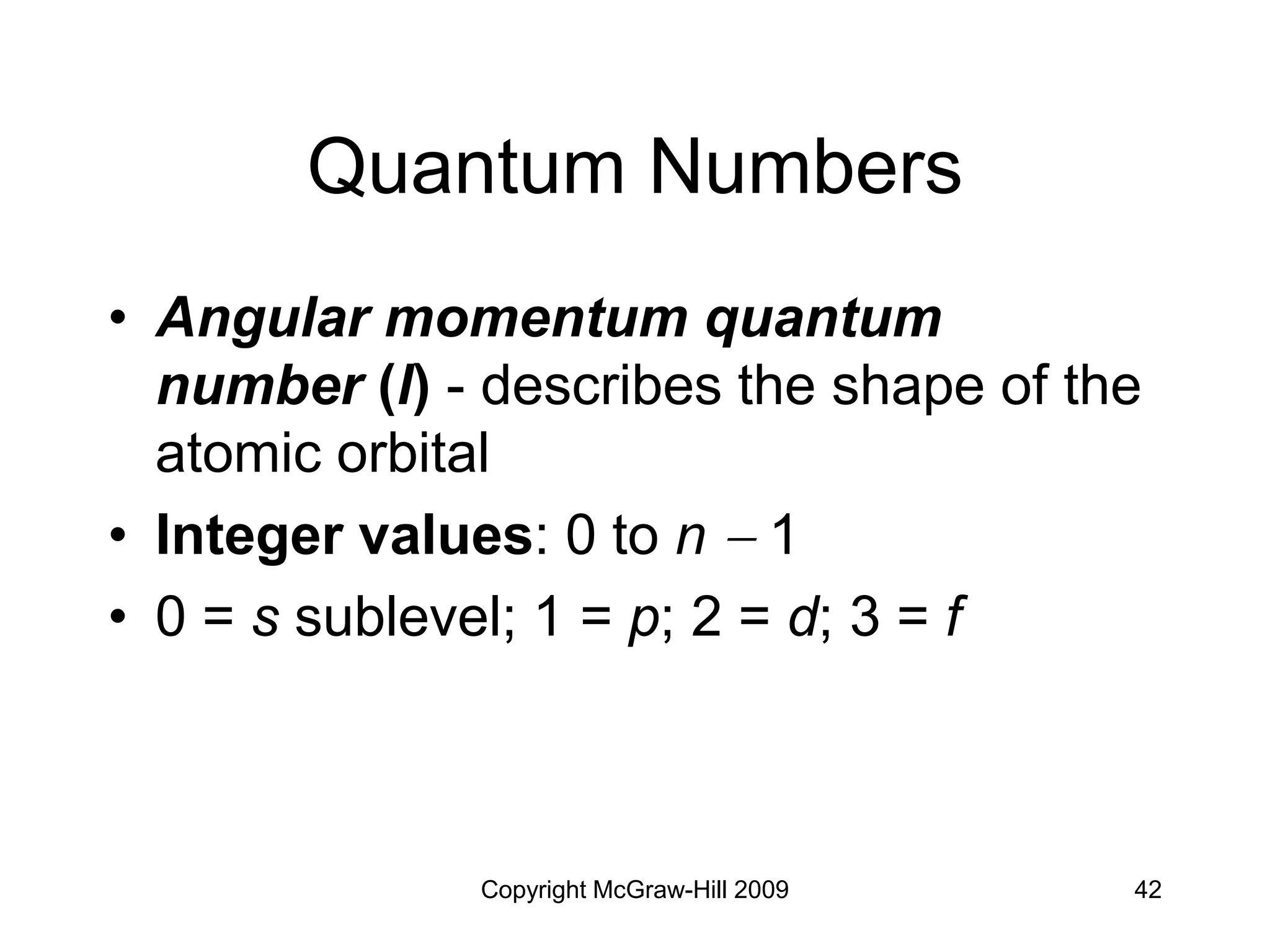
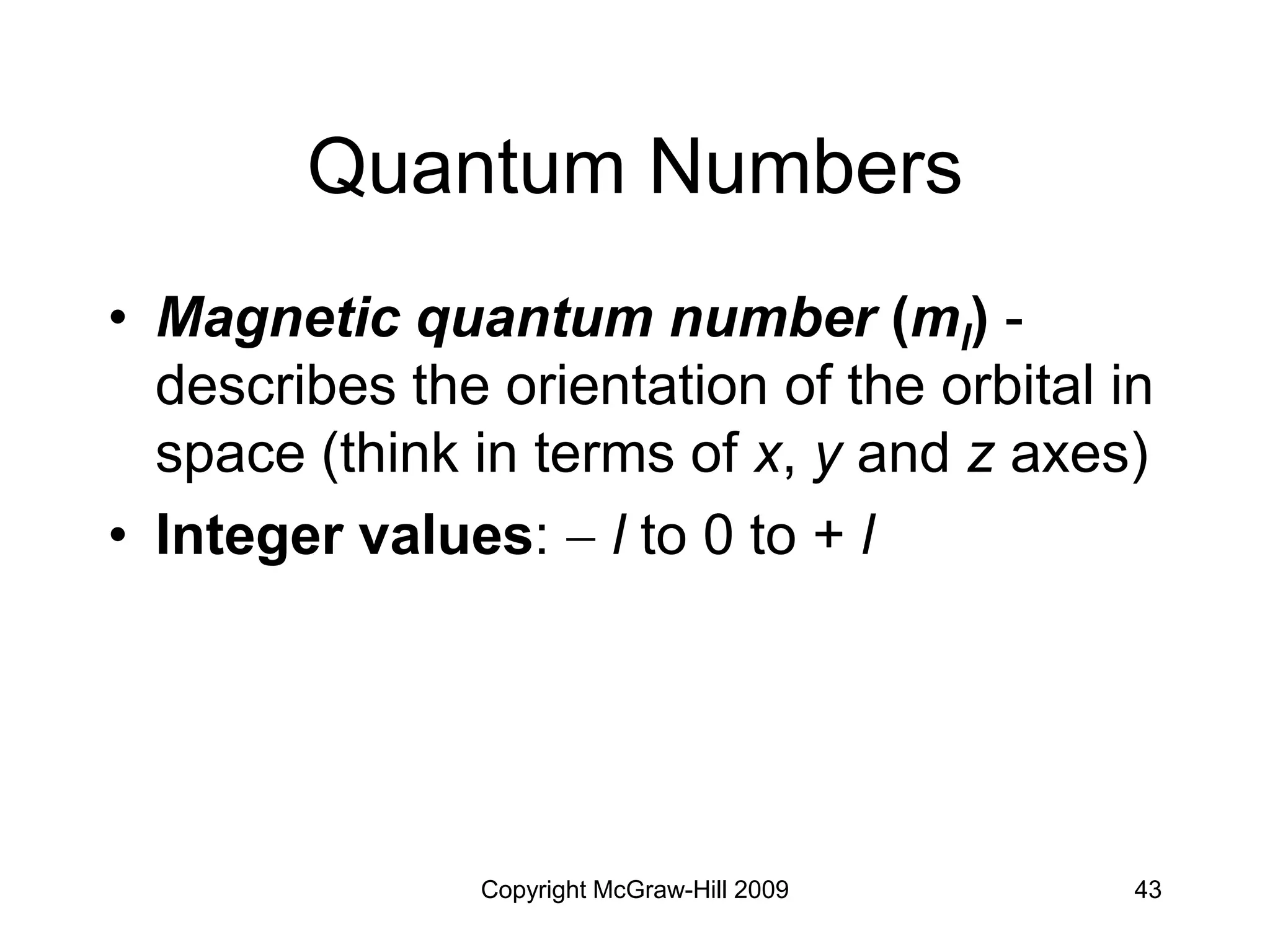
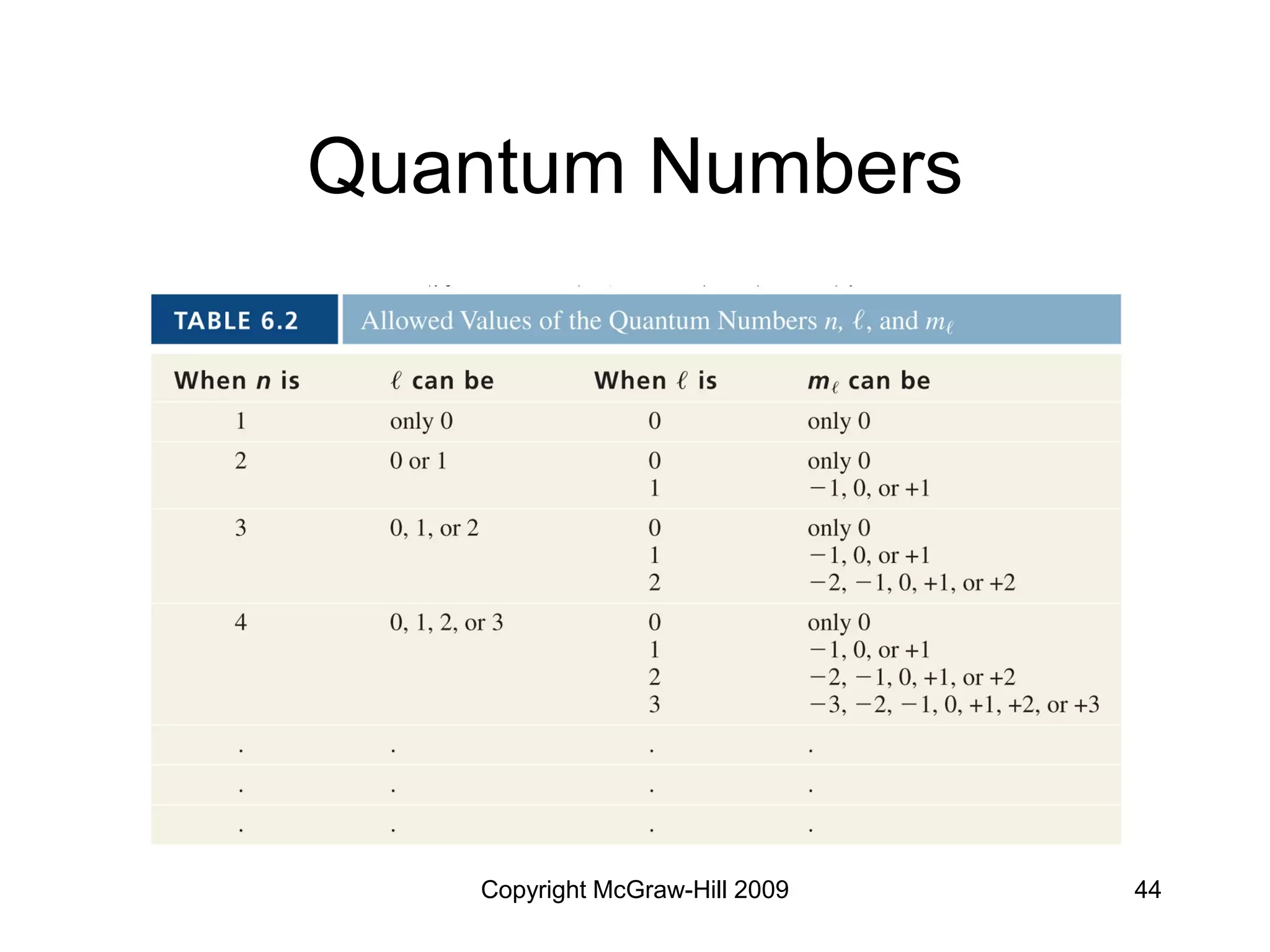
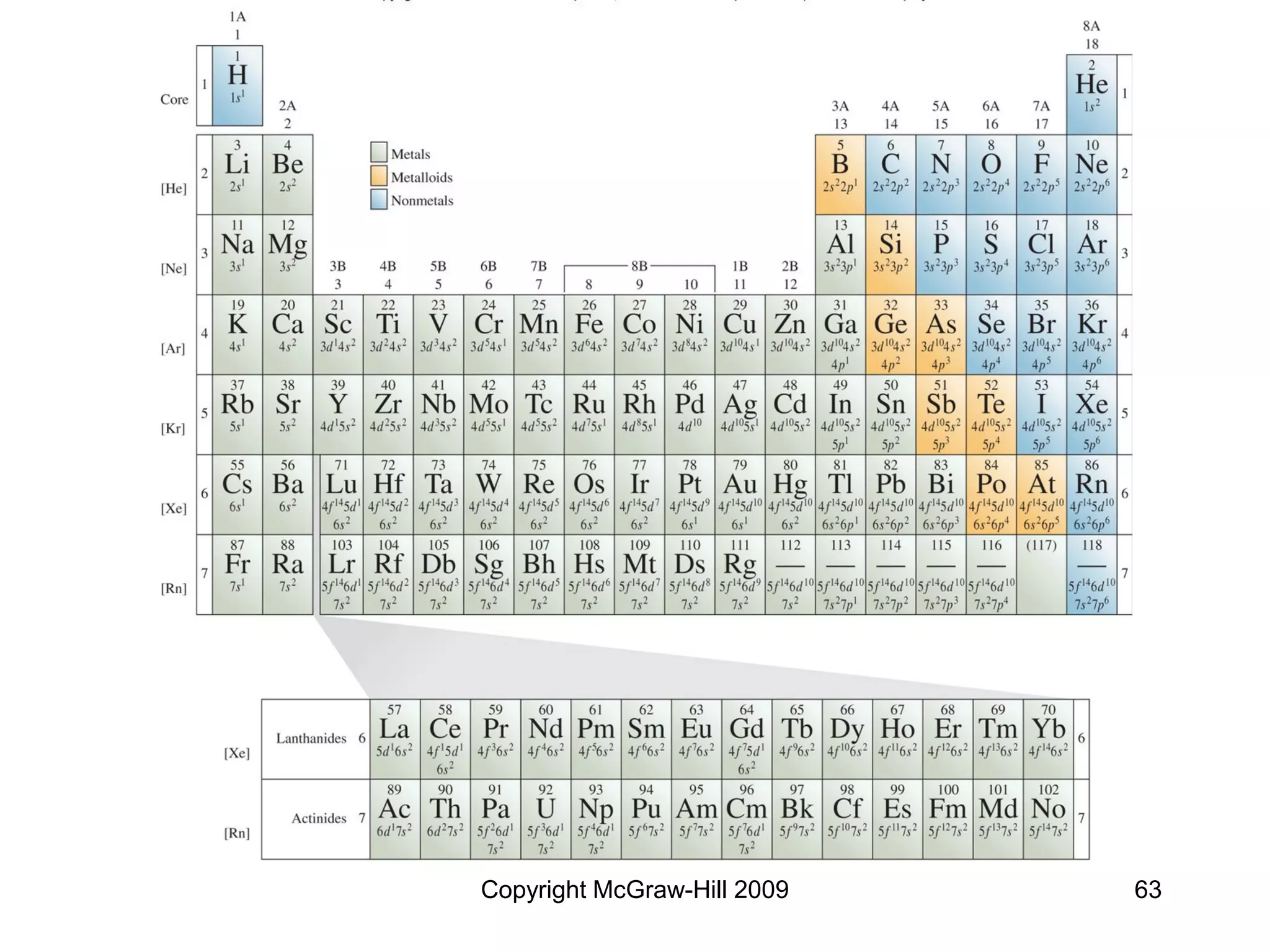
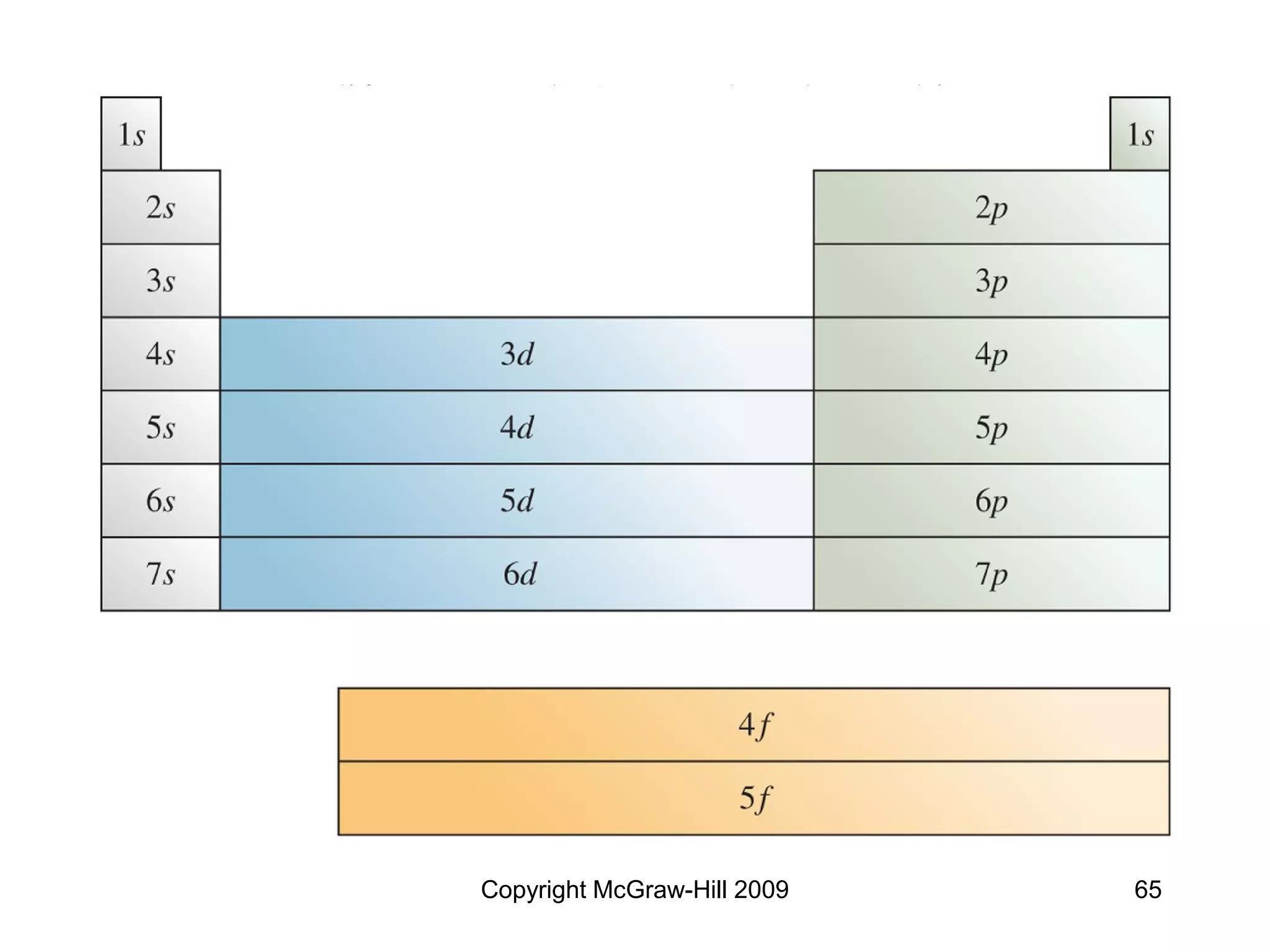
Electron Configurations and
the Periodic Table
• Noble gas core configuration - can be
used to represent all elements but H
and He
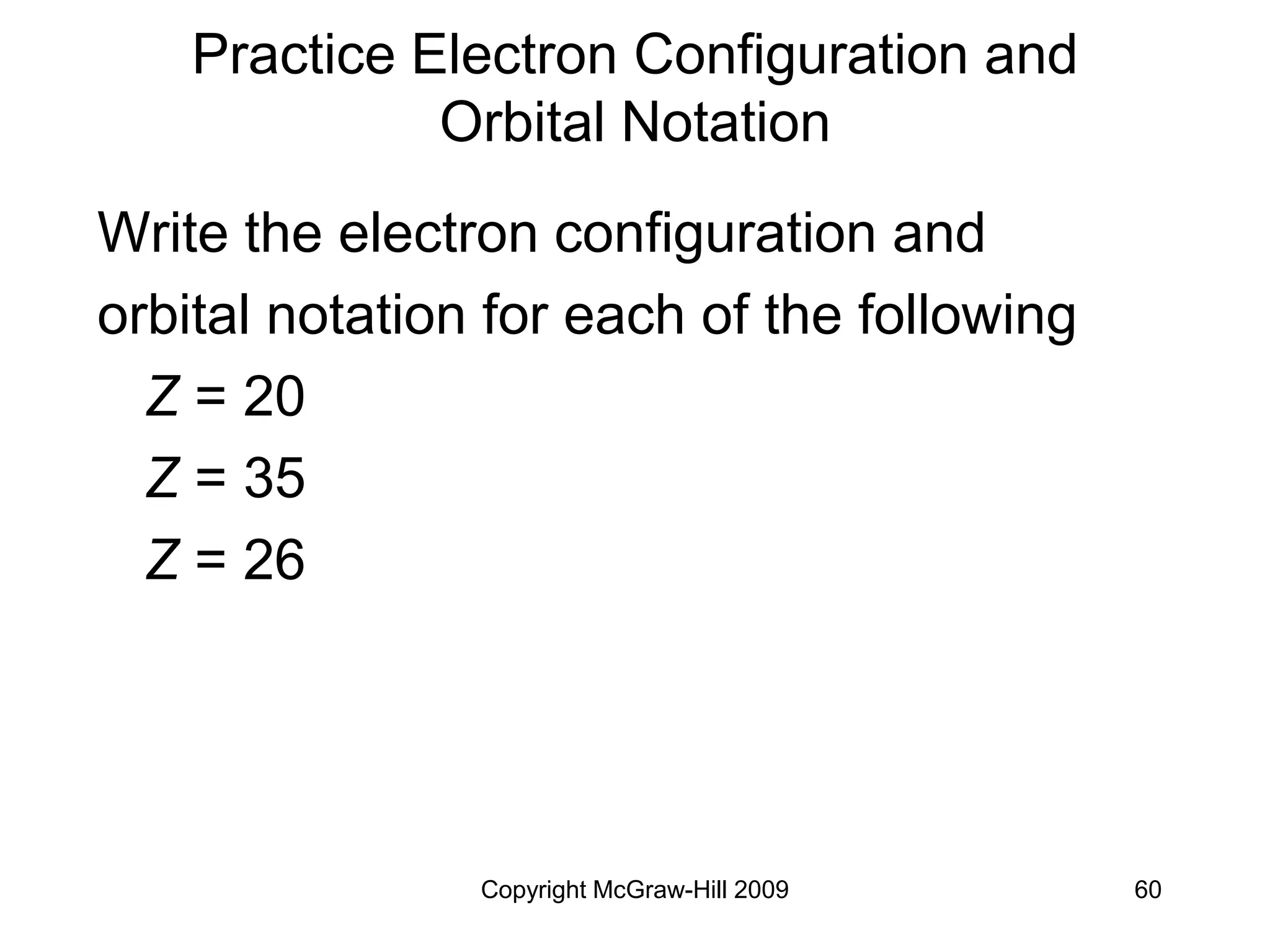
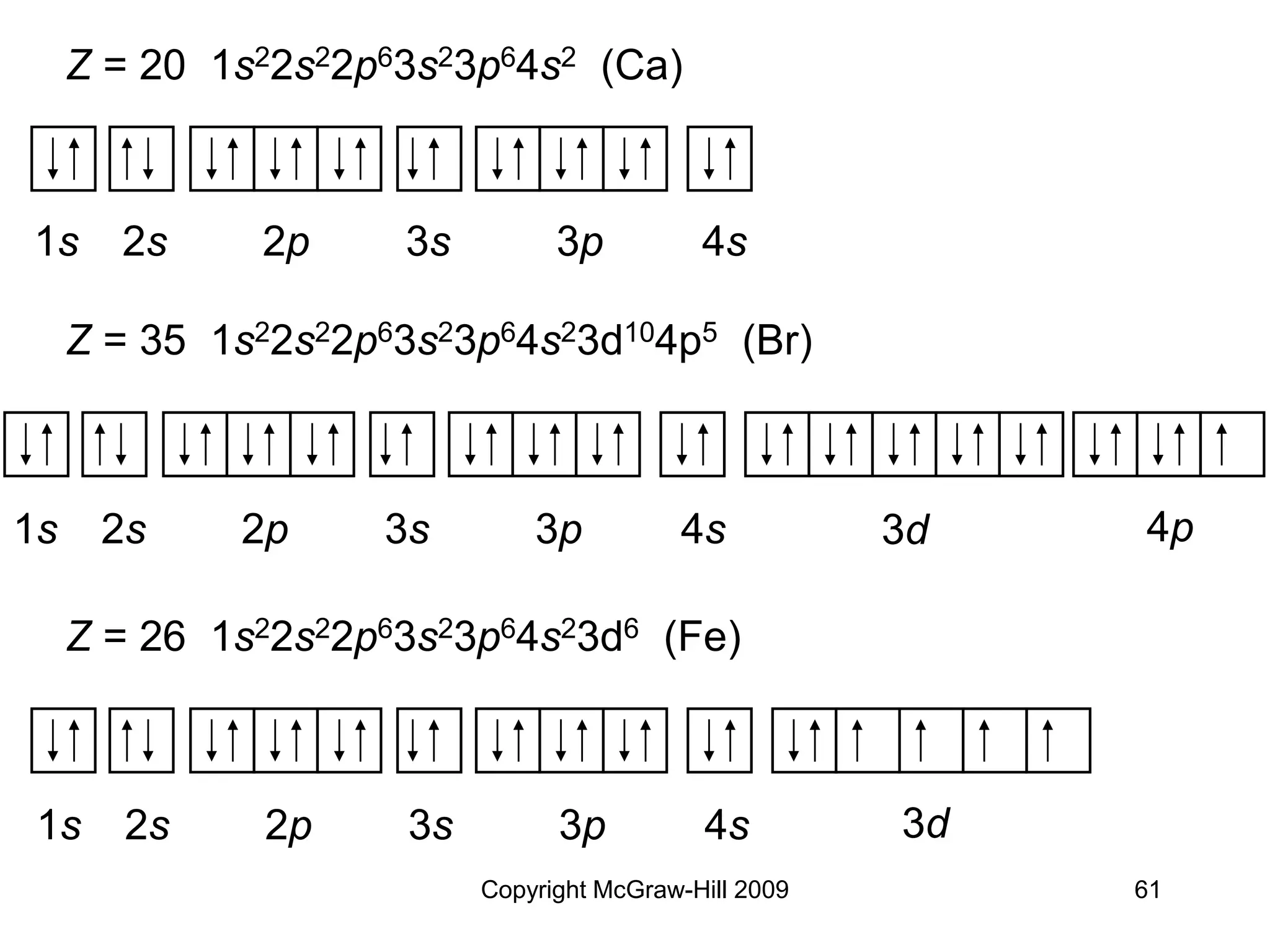
Example:
Z = 15 [1s22s22p6]3s23p3 (P)
[Ne] 3s23p3 (P)](https://image.slidesharecdn.com/ch6temp-230115173345-5d2fa277/75/Quantum-theory-and-electronic-structure-of-atom-64-2048.jpg)
![Copyright McGraw-Hill 2009 66
Too Good to Be True?
• Not all elements follow the “order” of the
diagonal rule
• Notable exceptions: Cu (Z = 29) and
Cr (Z = 24)
Cu = [Ar]4s13d10
Cr = [Ar]4s13d5
Reason: slightly greater stability associated with filled
and half-filled d subshells](https://image.slidesharecdn.com/ch6temp-230115173345-5d2fa277/75/Quantum-theory-and-electronic-structure-of-atom-66-2048.jpg)