This document contains a chapter from the textbook "Introduction to Engineering Thermodynamics" by Sonntag and Borgnakke. It provides the solution manual for chapter 4, which was authored by Claus Borgnakke. The chapter covers various thermodynamic concepts related to work, including poltropic processes, boundary work for multi-step processes, and rates of work. It includes over 70 example problems and their step-by-step solutions.









![Borgnakke
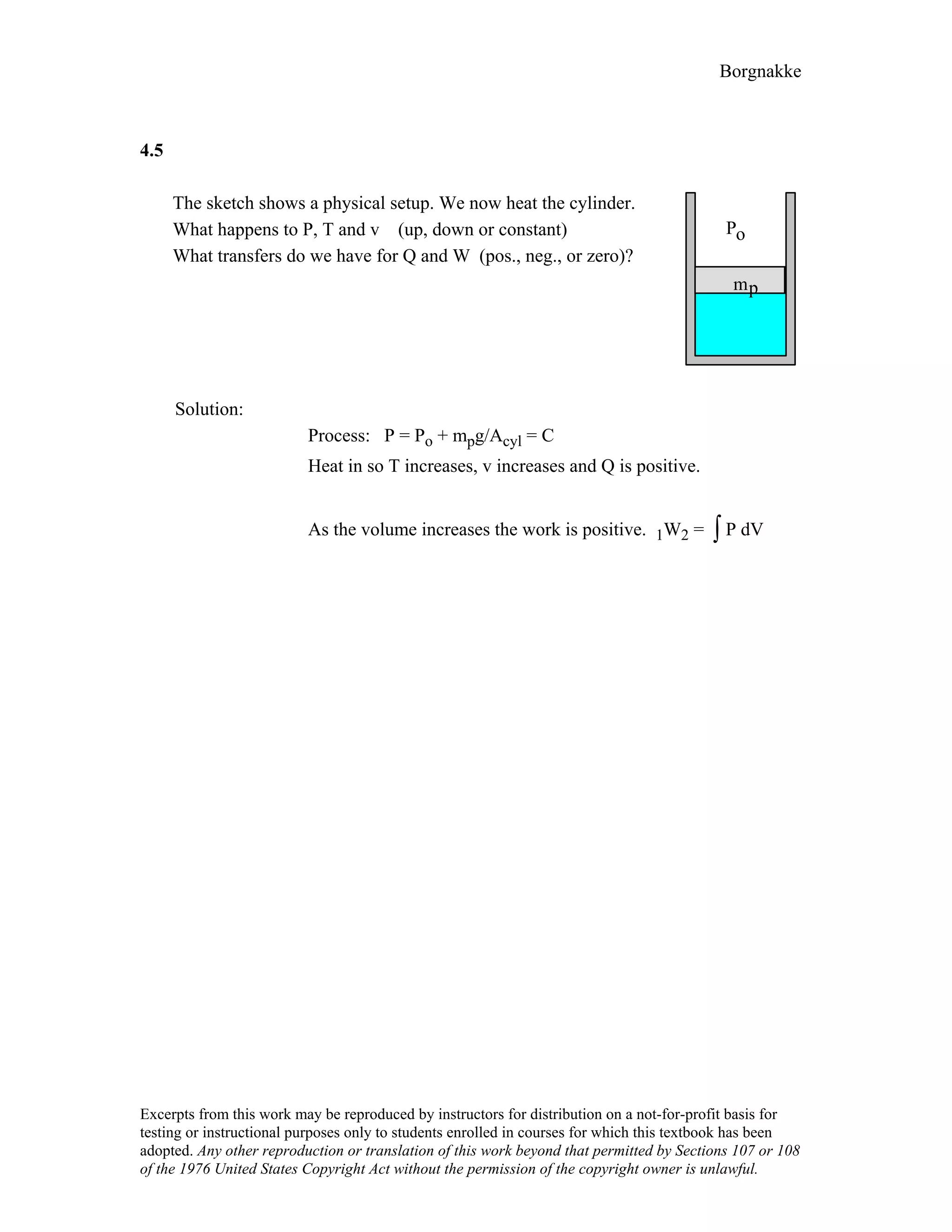
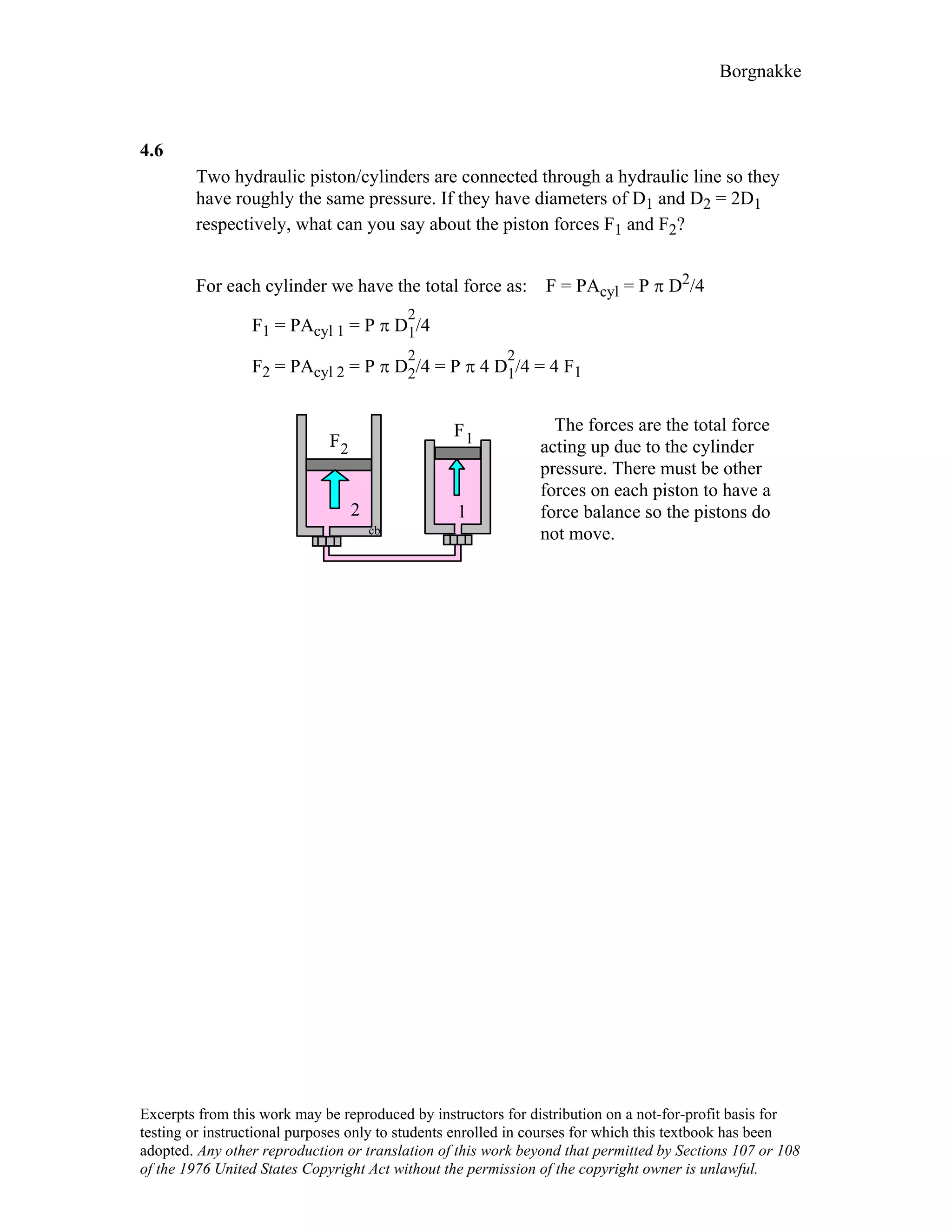
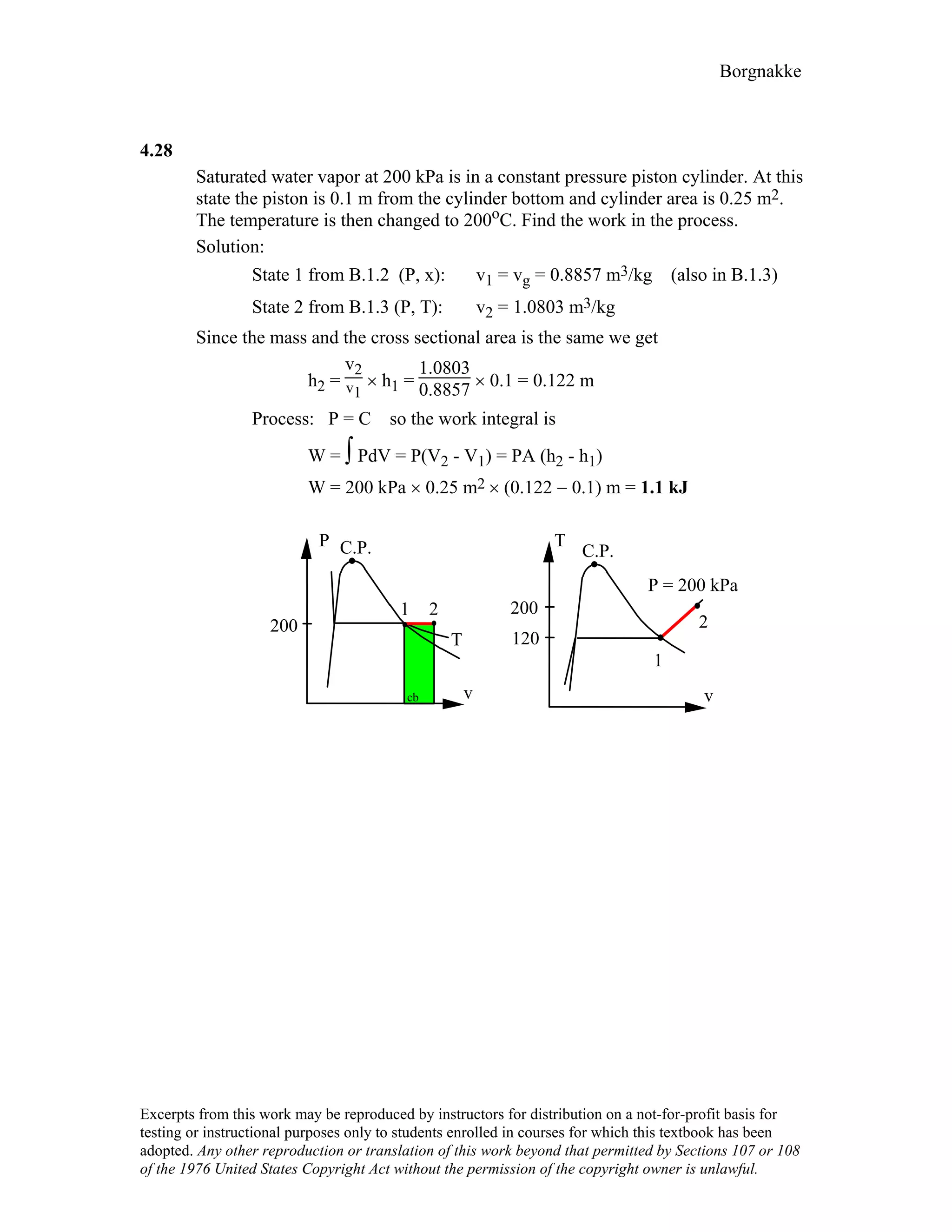
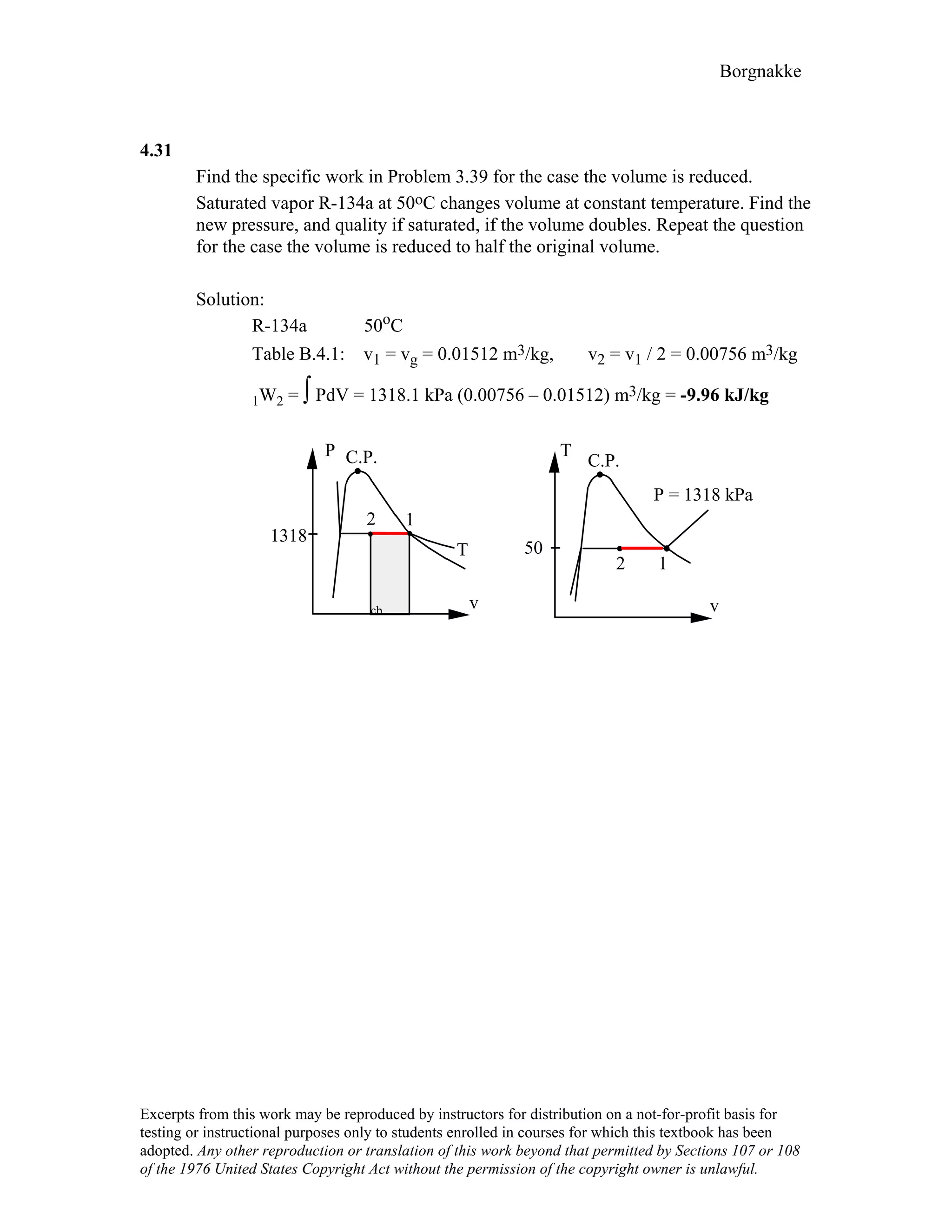
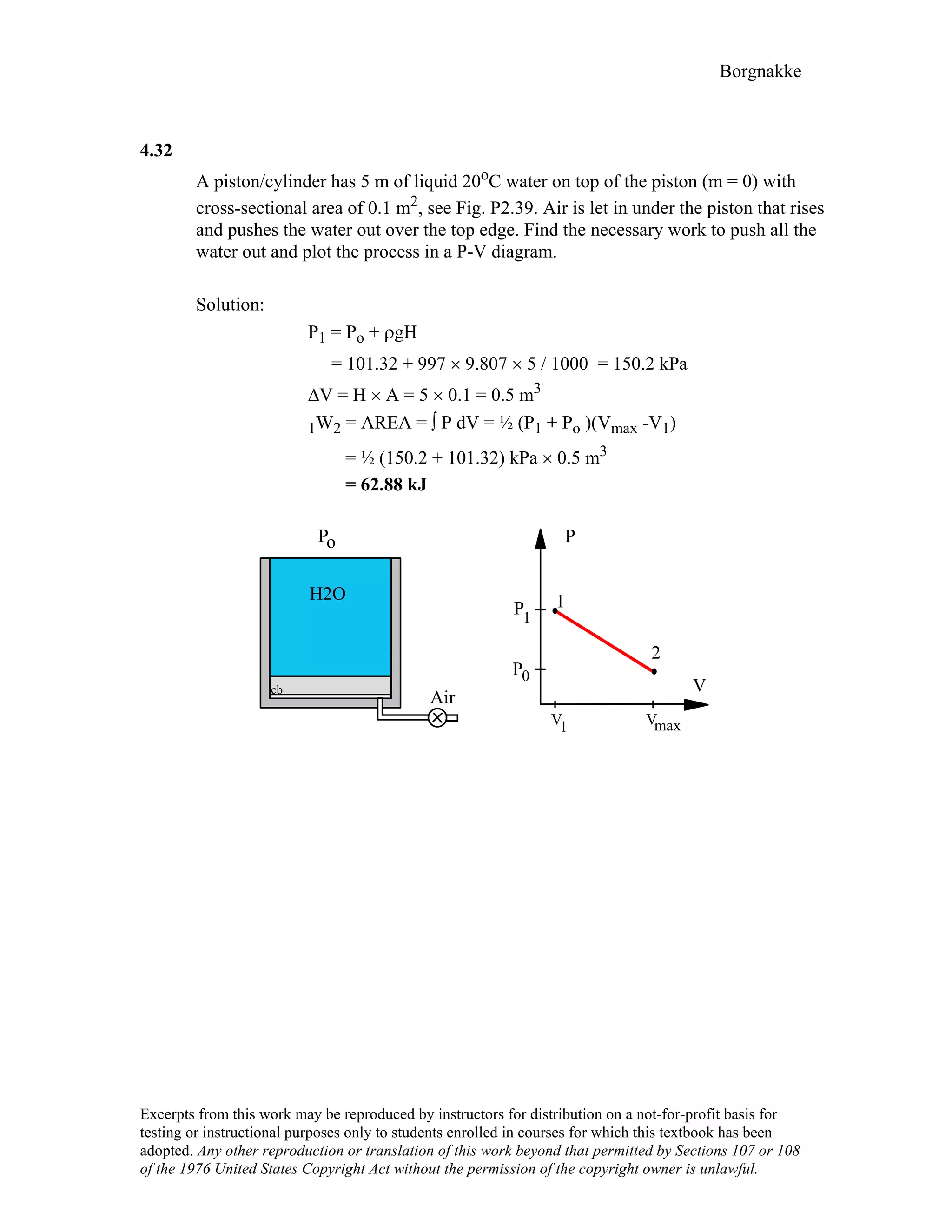
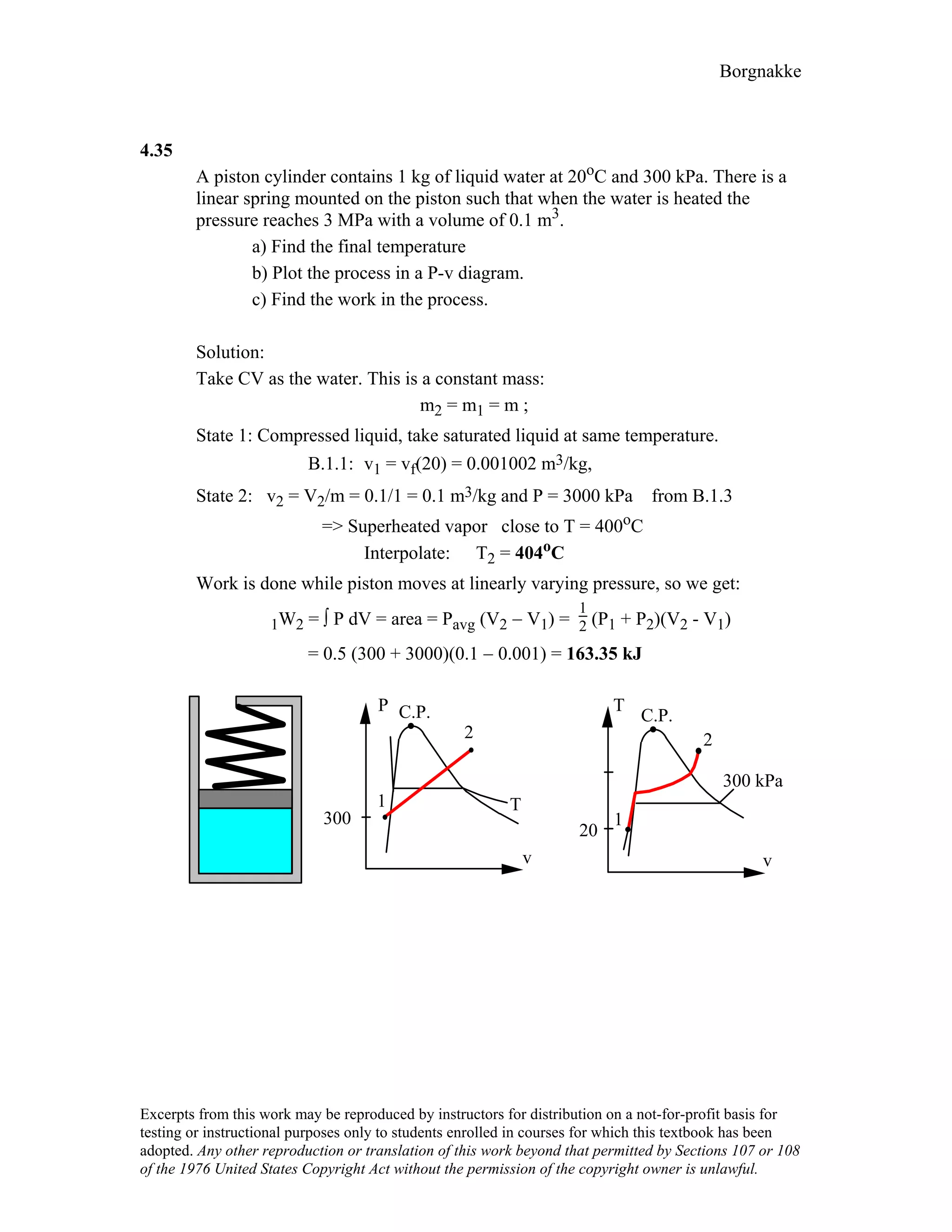
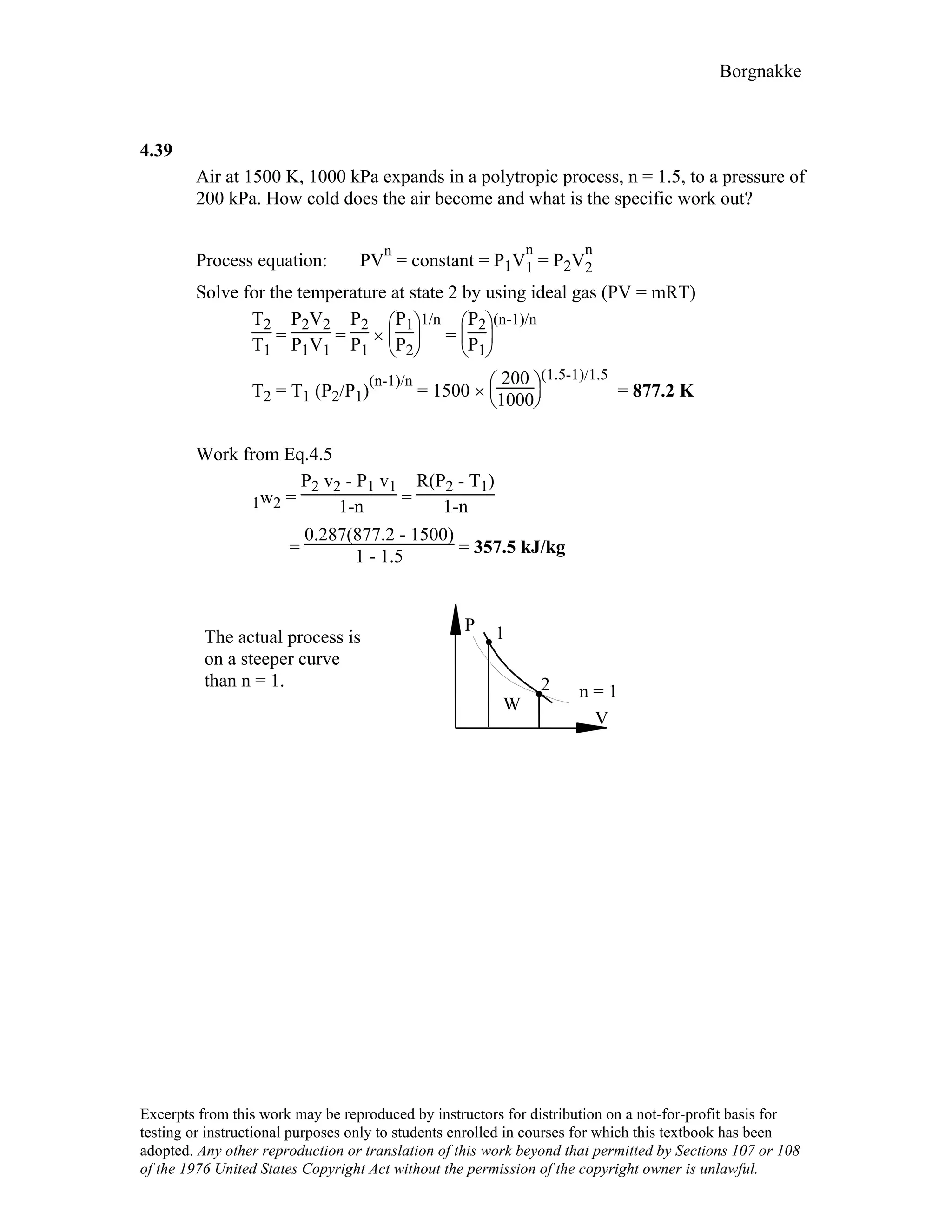
4.9
For the indicated physical set-up in a-b and c shown in Fig. P4.8 write a process
equation and the expression for work.
a) P = P1 and V ≥ Vstop or V = Vstop and P ≤ P1
1W2 = P1(V2 – V1) [ P1 = Pfloat ]
1
b) P = A + BV; 1W2 = 2 (P1 + P2)(V2 – V1)
c) P = P1 and V ≤ Vstop or V = Vstop and P ≥ P1
1W2 = P1(V2 – V1) [ P1 = Pfloat ]
a) b) c)
Po
mp
R-22
P P P
1
P 1
1
P 1
1 P
V 1
V V
V1
Vstop V1 Vstop
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.](https://image.slidesharecdn.com/ch4-answertothehomeworkproblem-111004190802-phpapp02/75/Ch4-answer-to-the-homework-problem-12-2048.jpg)













![Borgnakke
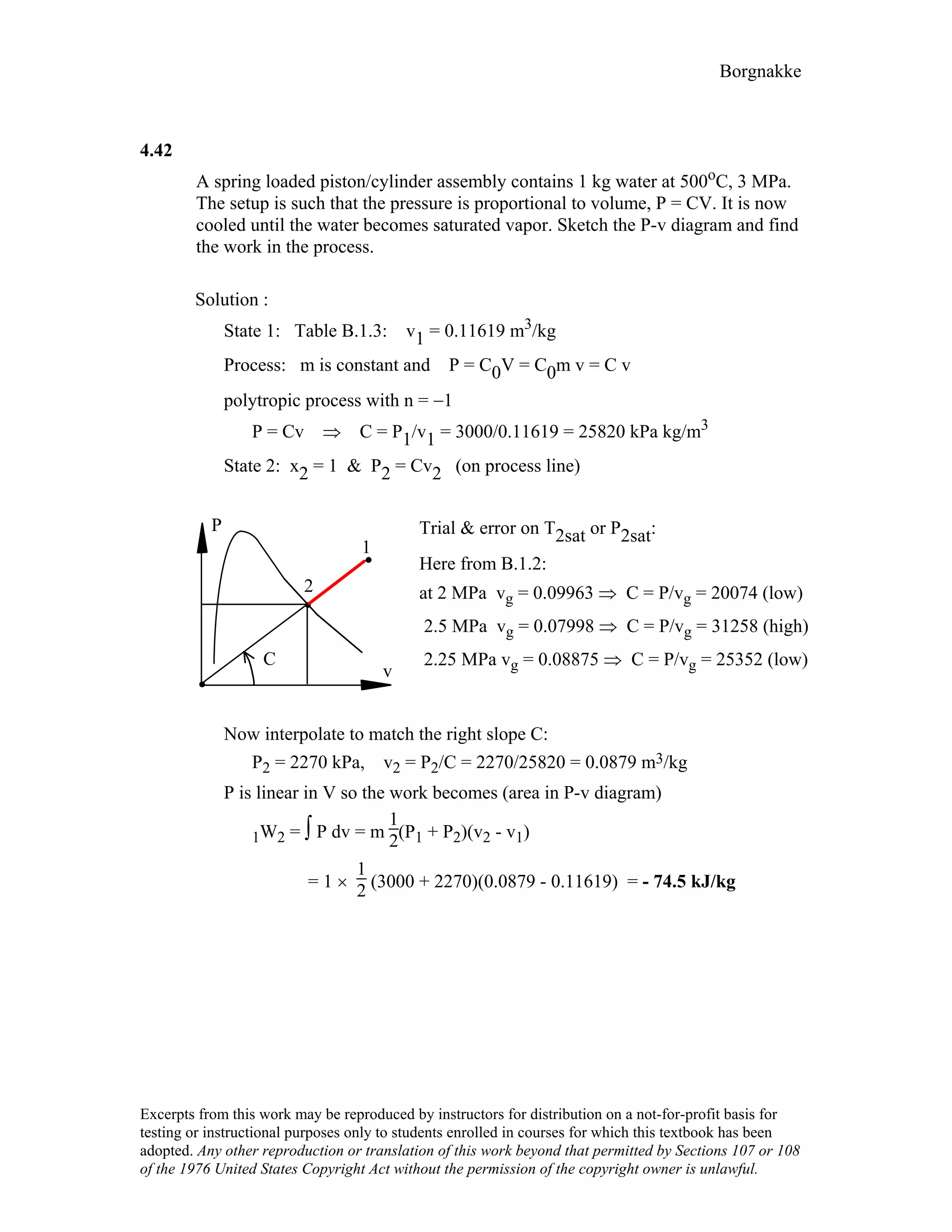
4.24
A steam radiator in a room at 25°C has saturated water vapor at 110 kPa flowing
through it, when the inlet and exit valves are closed. What is the pressure and the
quality of the water, when it has cooled to 25oC? How much work is done?
Solution: Control volume radiator.
After the valve is closed no more flow, constant volume and mass.
1: x1 = 1, P1 = 110 kPa ⇒ v1 = vg = 1.566 m3/kg from Table B.1.2
2: T2 = 25oC, ?
Process: v2 = v1 = 1.566 m3/kg = [0.001003 + x2 × 43.359] m3/kg
1.566 – 0.001003
x2 = 43.359 = 0.0361
State 2 : T2 , x2 From Table B.1.1 P2 = Psat = 3.169 kPa
1W2 = ⌠PdV = 0
⌡
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.](https://image.slidesharecdn.com/ch4-answertothehomeworkproblem-111004190802-phpapp02/75/Ch4-answer-to-the-homework-problem-27-2048.jpg)






















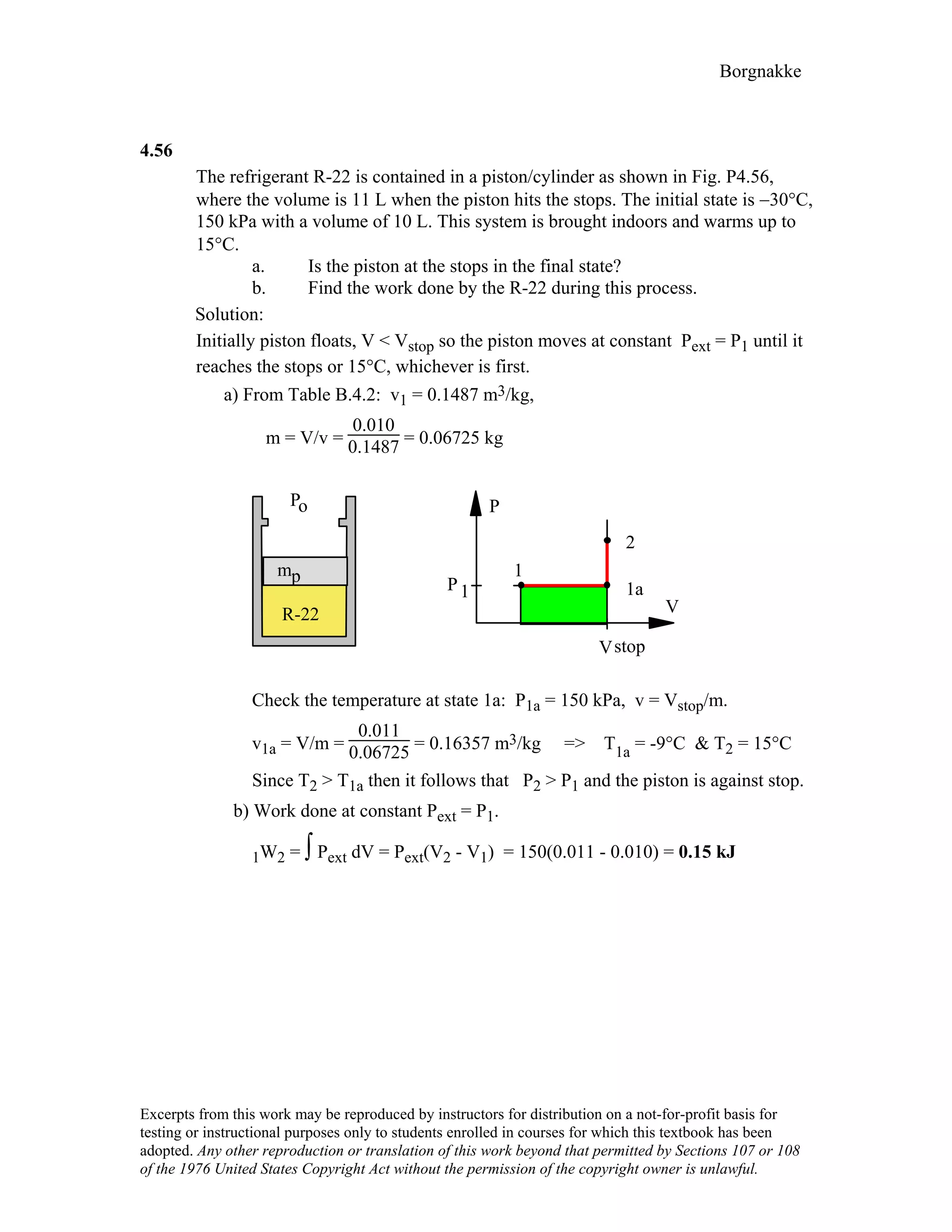


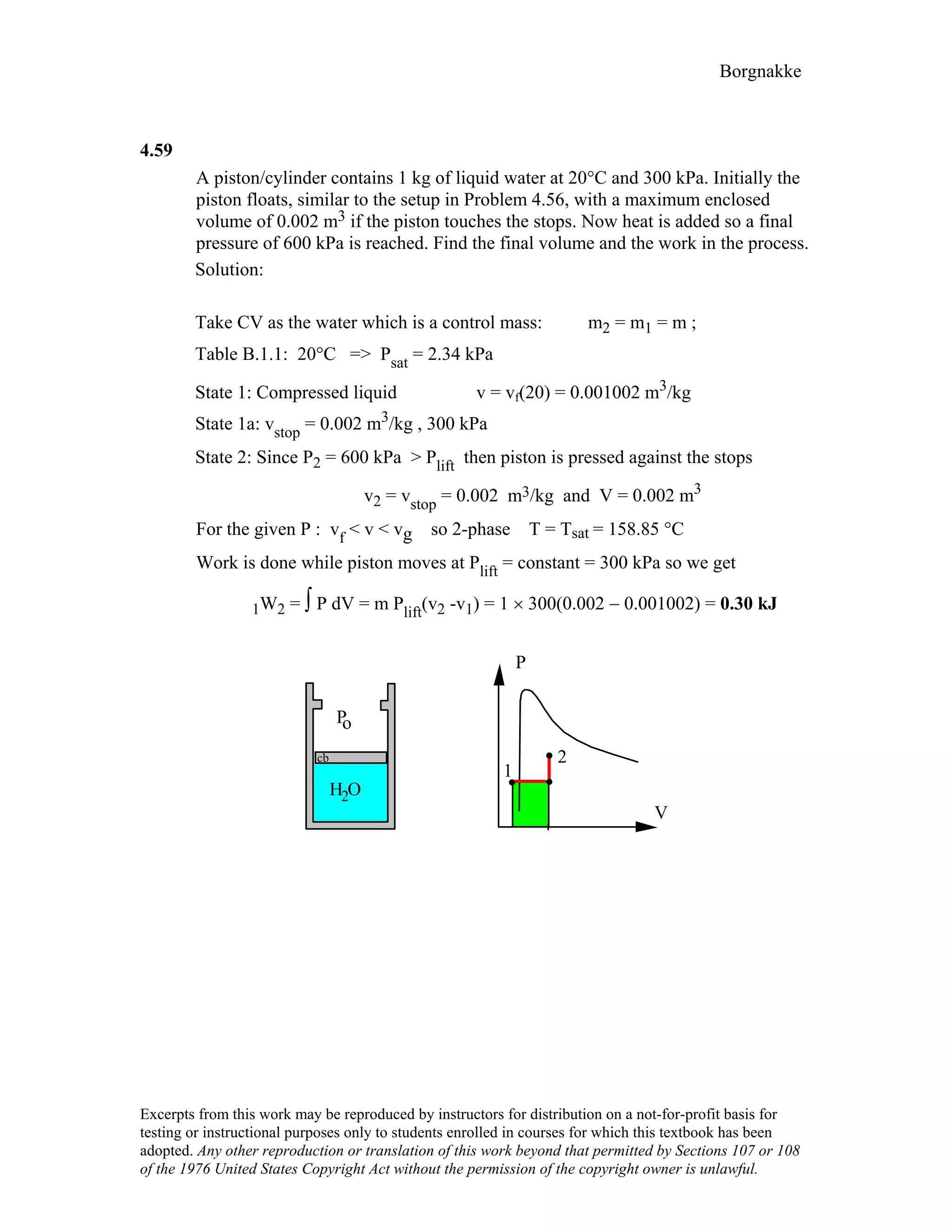


















![Borgnakke
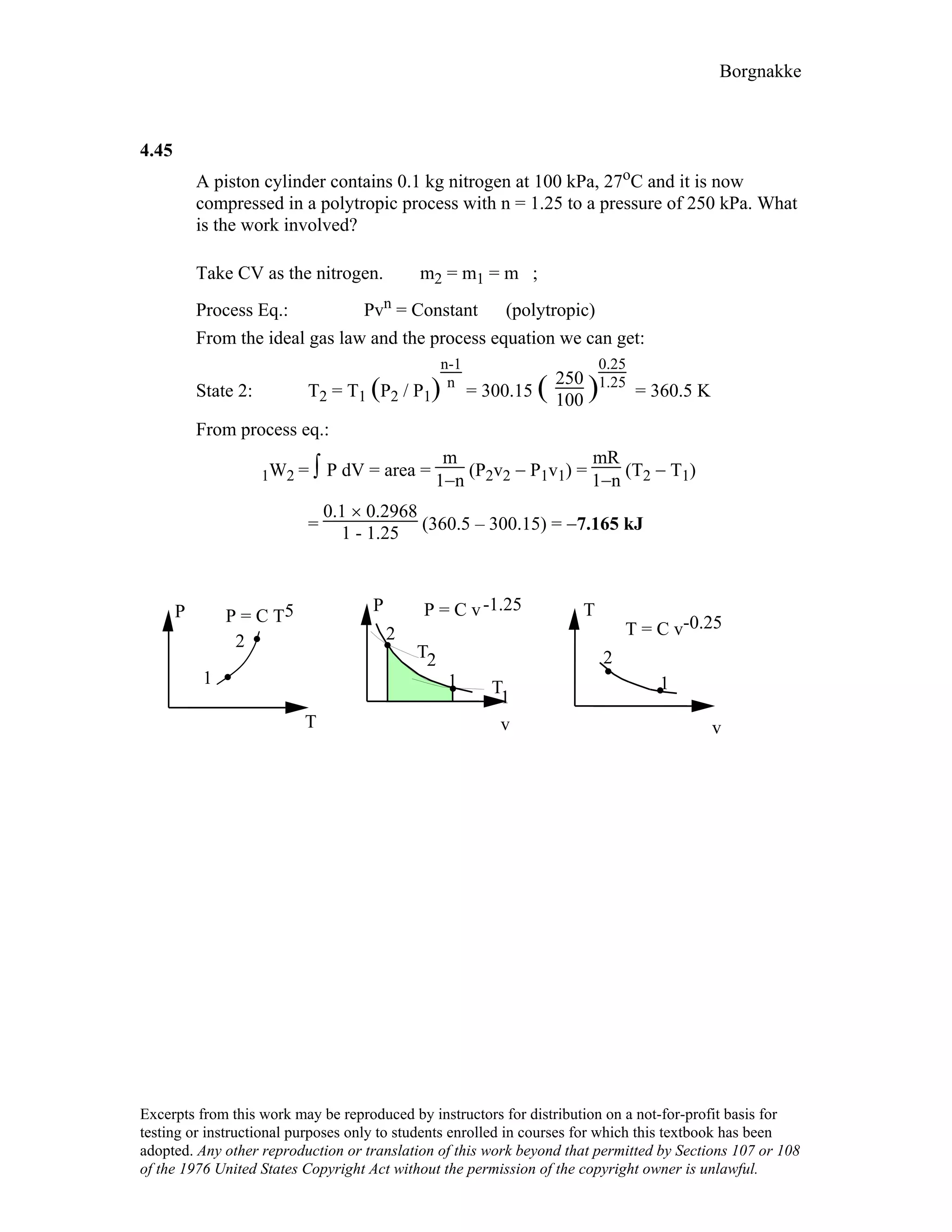
4.78
A battery is well insulated while being charged by 12.3 V at a current of 6 A.
Take the battery as a control mass and find the instantaneous rate of work and the
total work done over 4 hours.
Solution :
Battery thermally insulated ⇒ Q = 0
For constant voltage E and current i,
Power = E i = 12.3 × 6 = 73.8 W [Units V × A = W]
W = ∫ power dt = power ∆t
= 73.8 × 4 × 60 × 60 = 1 062 720 J = 1062.7 kJ
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.](https://image.slidesharecdn.com/ch4-answertothehomeworkproblem-111004190802-phpapp02/75/Ch4-answer-to-the-homework-problem-81-2048.jpg)












