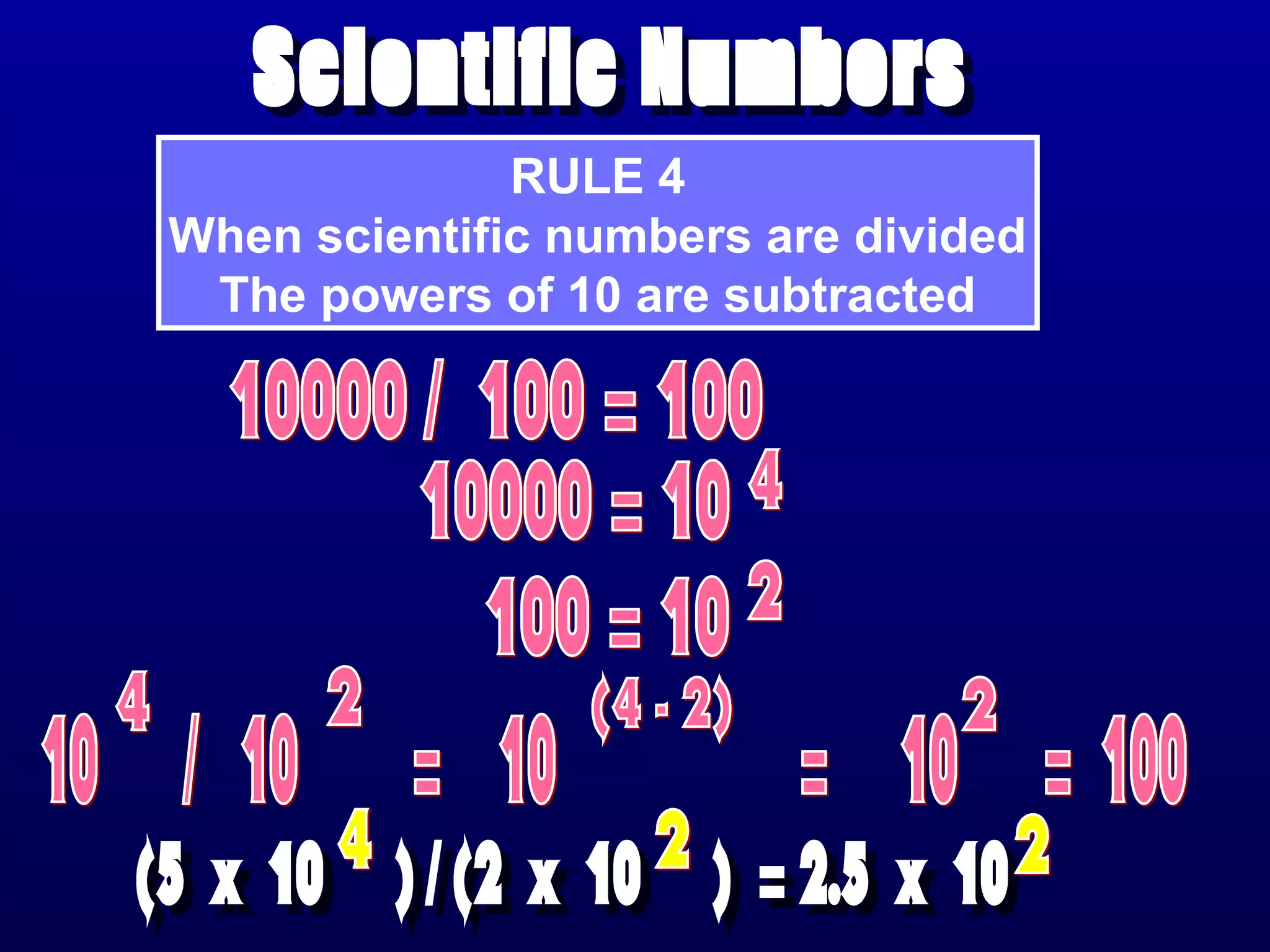
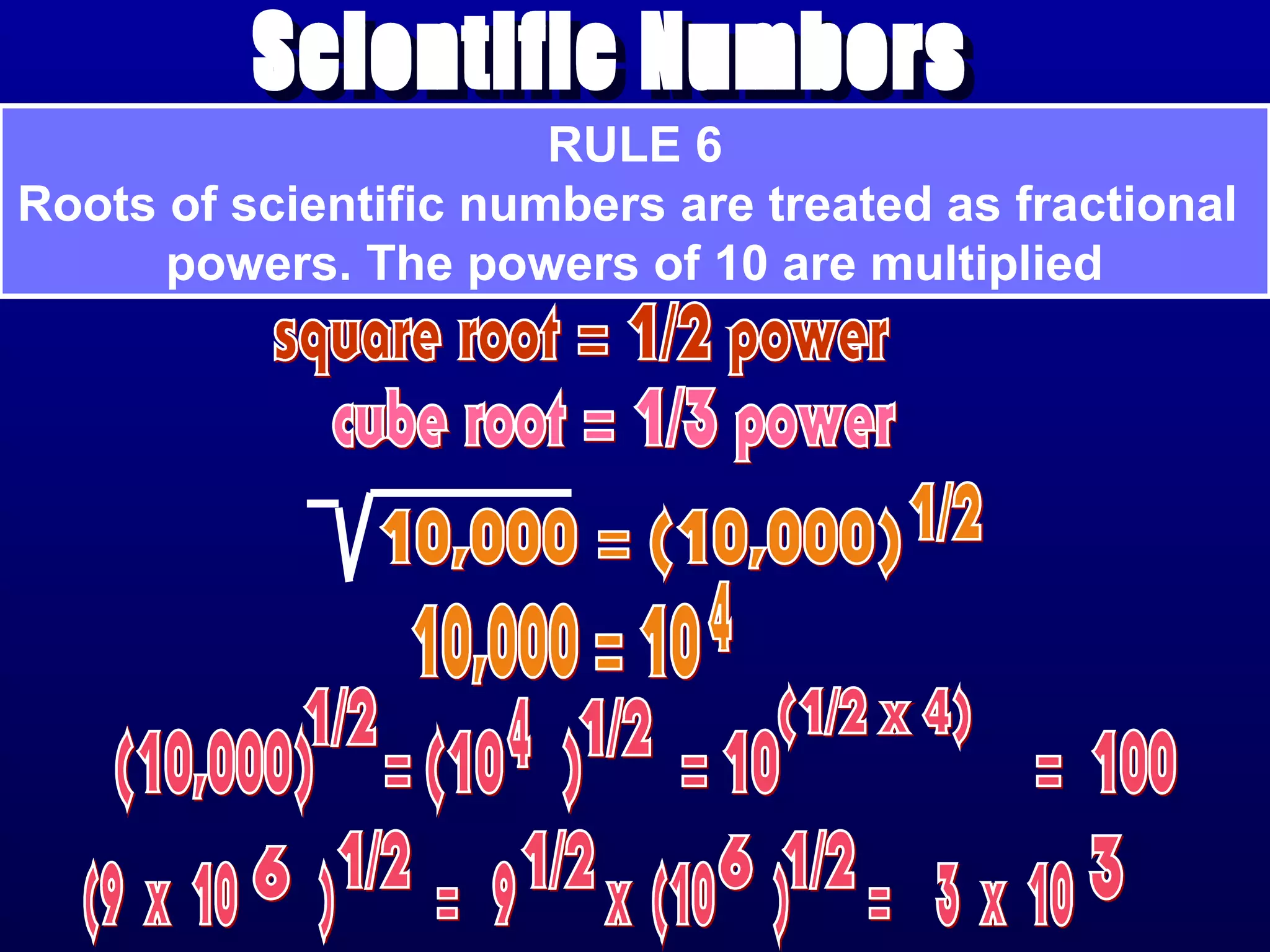
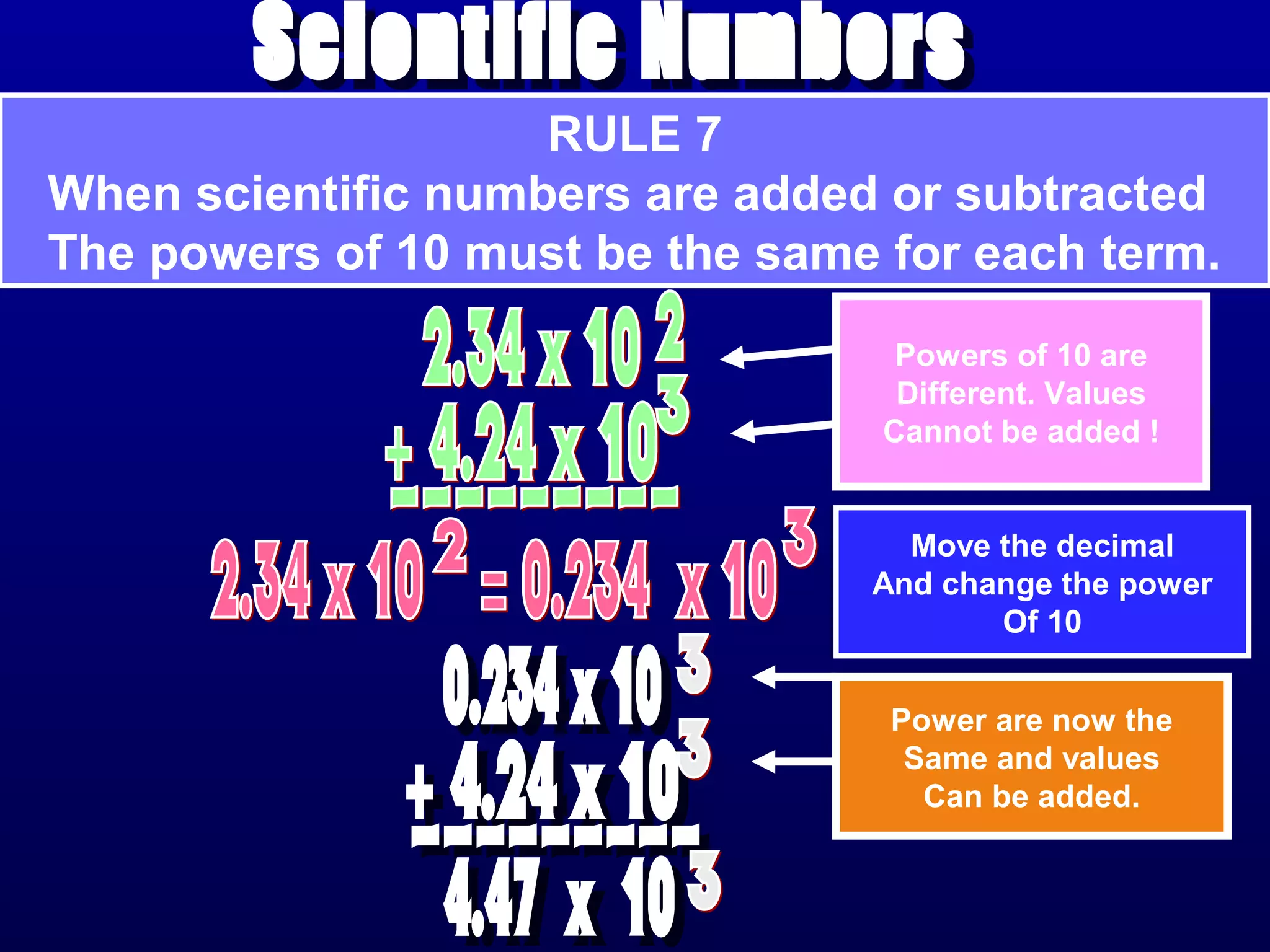
Here are the steps to write a number in standard form:
1. Locate the decimal point in the number.
2. Move the decimal point so that the number is greater than or equal to 1 but less than 10.
3. Count how many places the decimal was moved. If it was moved to the left, the exponent is positive. If it was moved to the right, the exponent is negative.
4. Write the number as the coefficient (the number with 1-9) and 10 with the exponent determined in step 3.
For example:
0.00045 would be written as 4.5 × 10-4
Since the decimal was moved 3 places to the right,

















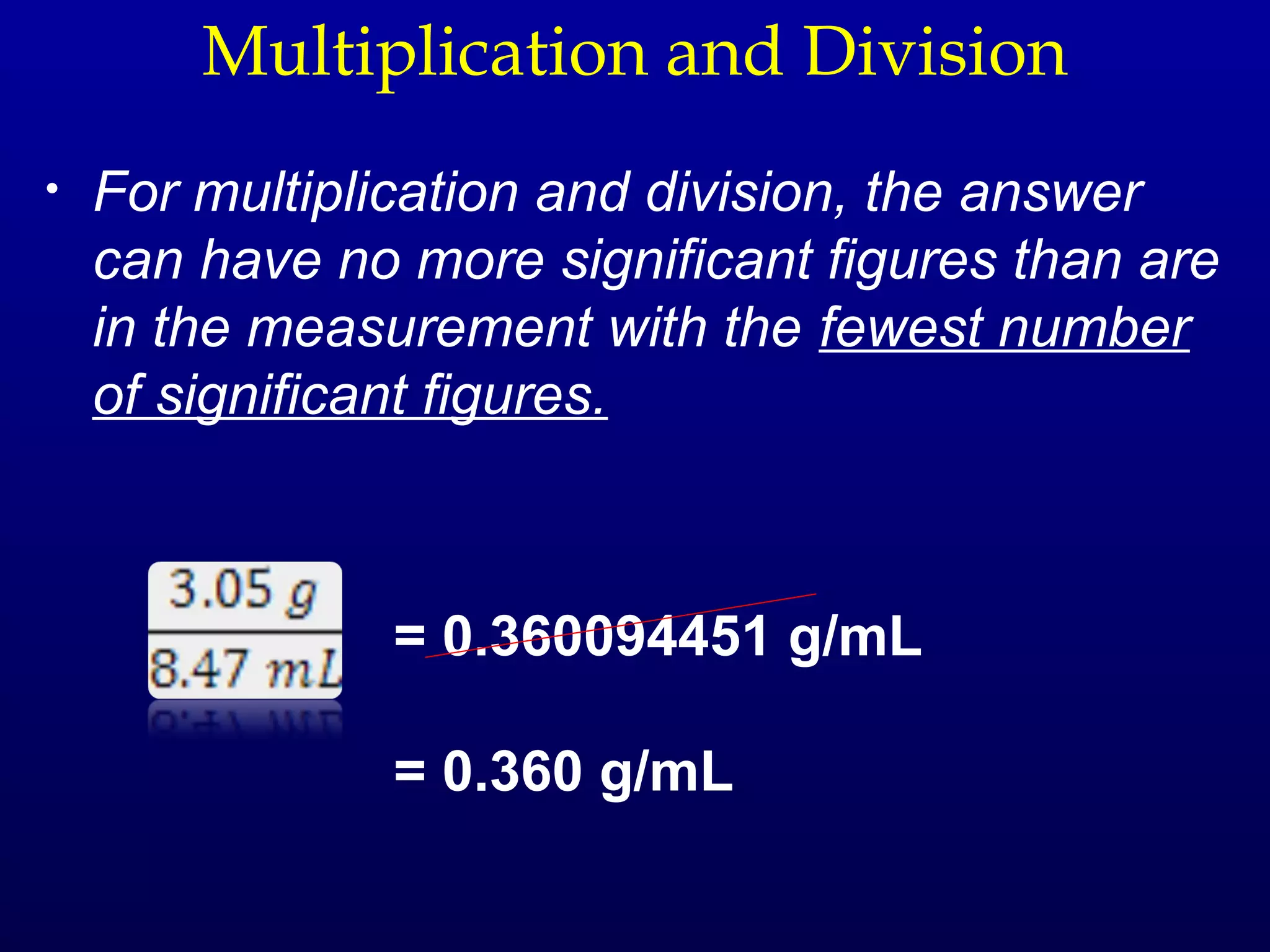
![Density
Density - The mass of a unit volume of a material.
density = mass/volume
What is the density of a cubic block of wood that is
2.4 cm on each side and has a mass of 9.57 g?
volume = [2.4 cm x 2.4 cm x 2.4 cm]
density = (9.57 g)/(13.8 cm3)
= 0.69 g/cm3 = 0.69 g/mL
Note that 1 cm3 = 1 mL](https://image.slidesharecdn.com/fmc1ooch1-121218114126-phpapp02/75/measurements-20-2048.jpg)