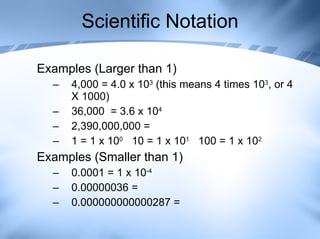
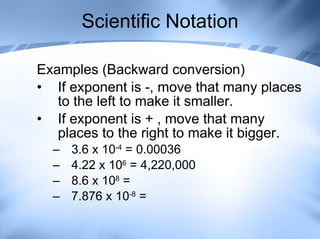
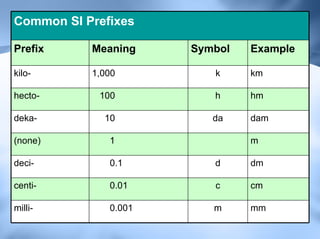
The document discusses measurement and conversions using the International System of Units (SI). It defines the base SI units for length, volume, mass, temperature and time. It also describes the common SI prefixes like kilo, hecto and milli used to indicate multiplication or division of the base units by powers of ten. Examples are provided to show how to convert between different units using the prefix multipliers.










![Metric System Summary Metric (SI) units are used almost entirely throughout science and will be used in this course! Length—units are meters Volume—units are liters Mass—grams Prefixes in order of largest to smallest (k) (h) (da) (d) (c) (m) kilo hecto deca Base deci centi milli 1000 100 10 [meter] 0.1 0.01 0.001 [liter] [gram]](https://image.slidesharecdn.com/measurementconversions-110815102649-phpapp02/85/Measurement-conversions-12-320.jpg)