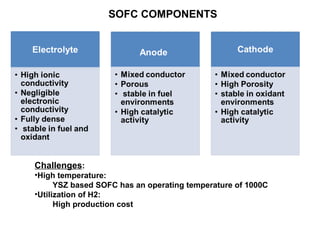
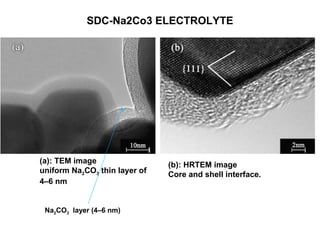
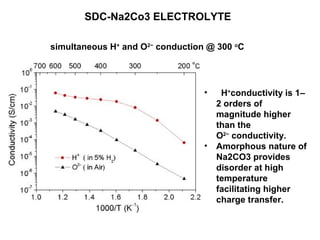
The document discusses the use of ceramic nanocomposites in solid oxide fuel cells (SOFCs), emphasizing their high energy efficiency, low emissions, and ability to lower operating temperatures. It highlights the development of composite electrolytes, specifically the SDC-Na2CO3 nanocomposite, which improves ionic conductivity and enables simultaneous proton and oxygen ion conduction at lower temperatures. The conclusion stresses the importance of developing cheaper materials for commercialization and the potential for using crude hydrocarbon fuels in this environmentally friendly technology.