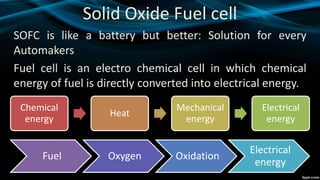
Solid oxide fuel cells (SOFCs) directly convert chemical energy from fuels into electrical energy through electrochemical reactions. SOFCs operate at high temperatures between 600-1800°C using fuels like natural gas, methane or propane. The electrolyte is made of ceramic materials like zirconium and doped perovskite. SOFCs have efficiencies between 45-60% and costs around 2700 INR/KW. They produce clean energy, have modular construction and long lifetimes up to 100,000 hours. Challenges include reducing costs through lower material costs and operating temperatures while increasing power outputs.