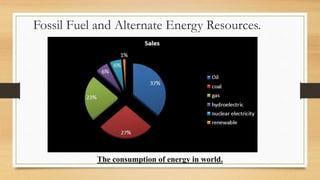
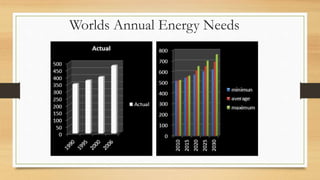
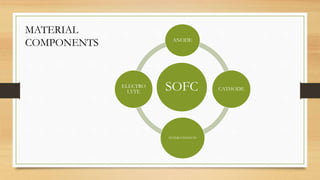
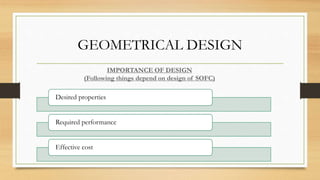
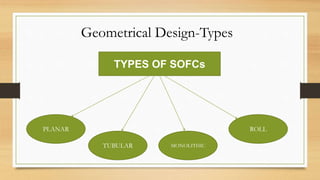
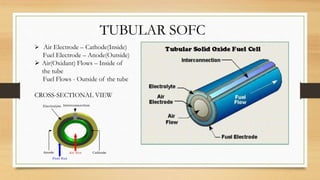
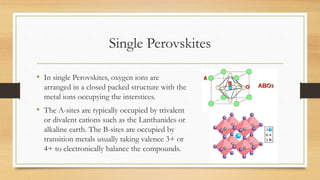
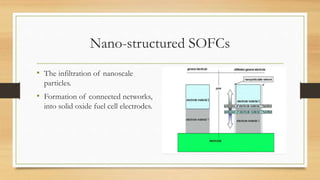
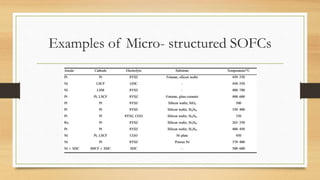
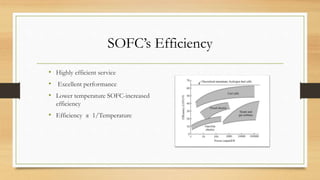
The document presents an overview of solid oxide fuel cells (SOFCs) as a clean and efficient energy source necessary due to the depletion of non-renewable energy resources. It discusses the functioning of fuel cells, their geometrical designs, materials used, and advancements for improving their applications. The conclusion emphasizes the potential of SOFCs as an alternative power source, highlighting their advantages and disadvantages.