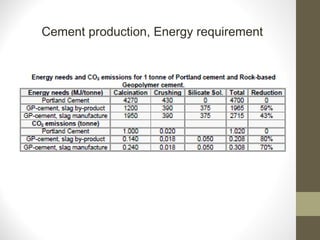
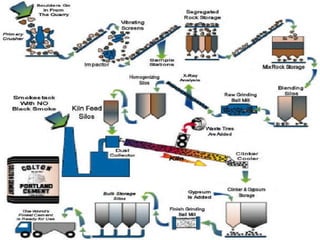
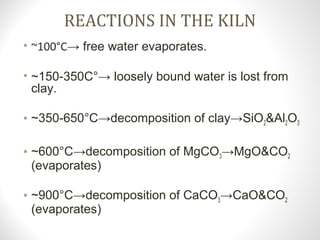
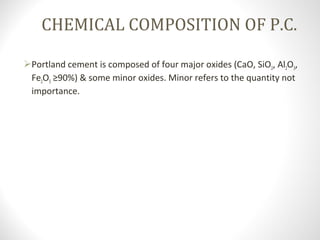
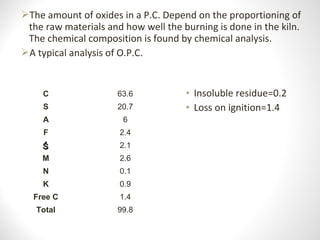
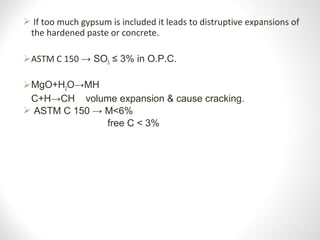
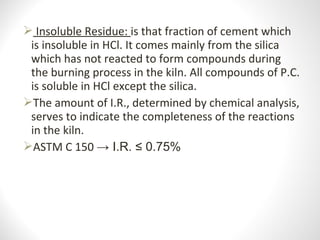
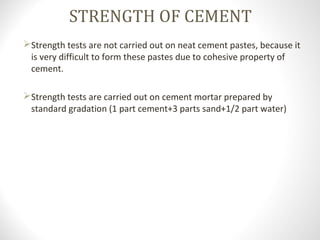
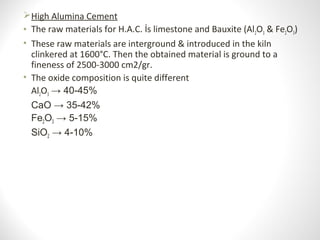
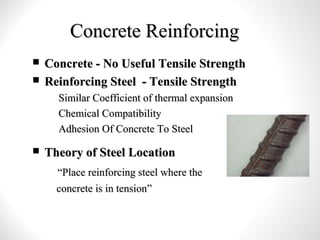
Cement is a binder that sets and hardens, binding other materials together. There are two main types: hydraulic cement, which sets in the presence of water, and non-hydraulic cement, which requires dry conditions to set. Portland cement is the most common type of hydraulic cement, produced by heating limestone, clay, and other materials in a kiln to form clinker, which is then ground with gypsum. The clinker is composed mainly of calcium silicates, aluminates, and aluminoferrites which give Portland cement its strength and properties.















































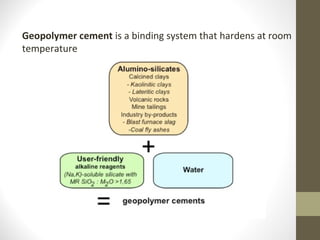
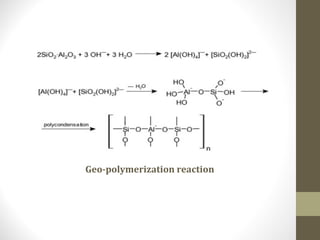
![Chemical structure and geopolymerization of geopolymer
cement
The geopolymerization process involves a substantially fast
chemical reaction under alkaline condition on Si-Al minerals
that result in a three dimensional polymeric chain and ring
structure consisting of Si-O-Al-O bonds, as follows:
There are three steps involved in geopolymerisation, i.e.,
dissolution of Al and Si in the alkali medium,
orientation, and polycondensation.
The dissolution and hydrolysis reactions are:
Al2O3 + 3H2O +2OH-
2[Al(OH)4]-
SiO2 + H2O + OH-
[SiO(OH)3]-
SiO2+ 2OH-
[SiO2(OH)2]2-
(in presence of strong alkali)](https://image.slidesharecdn.com/ibtechict2018cement-190126162542/85/cement-69-320.jpg)