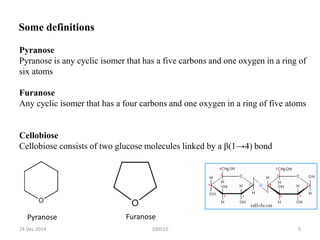
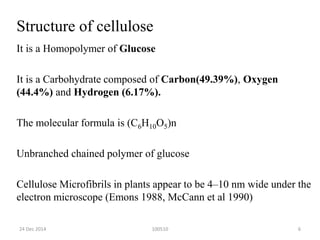
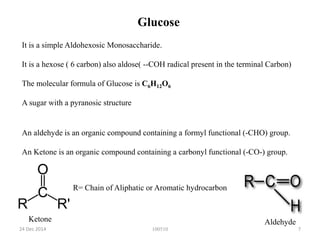
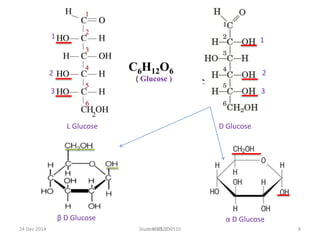
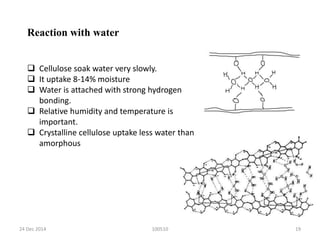
The document discusses cellulose, including its structure, properties, production in plants, and uses. Some key points:
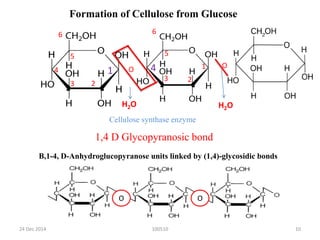
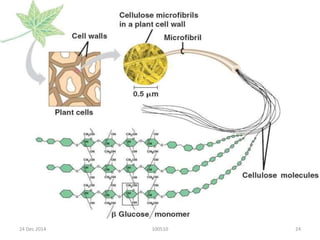
- Cellulose is the most abundant organic substance on Earth and is made of linear chains of glucose molecules linked together.
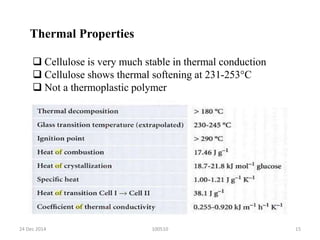
- It has a crystalline structure that gives it strength and it forms microfibrils in plant cell walls.
- Plants produce cellulose at their plasma membranes using enzyme complexes that spin the cellulose chains.
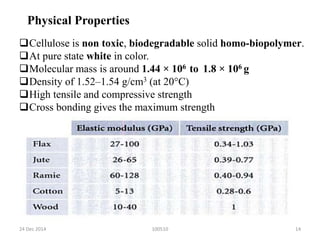
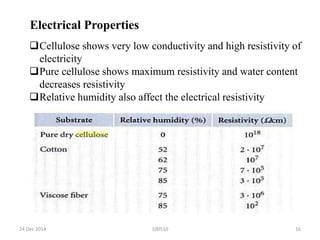
- Cellulose is strong, stable, and insoluble but can absorb some water. It is used to make products like cotton, paper, cellophane, and cellulose derivatives.