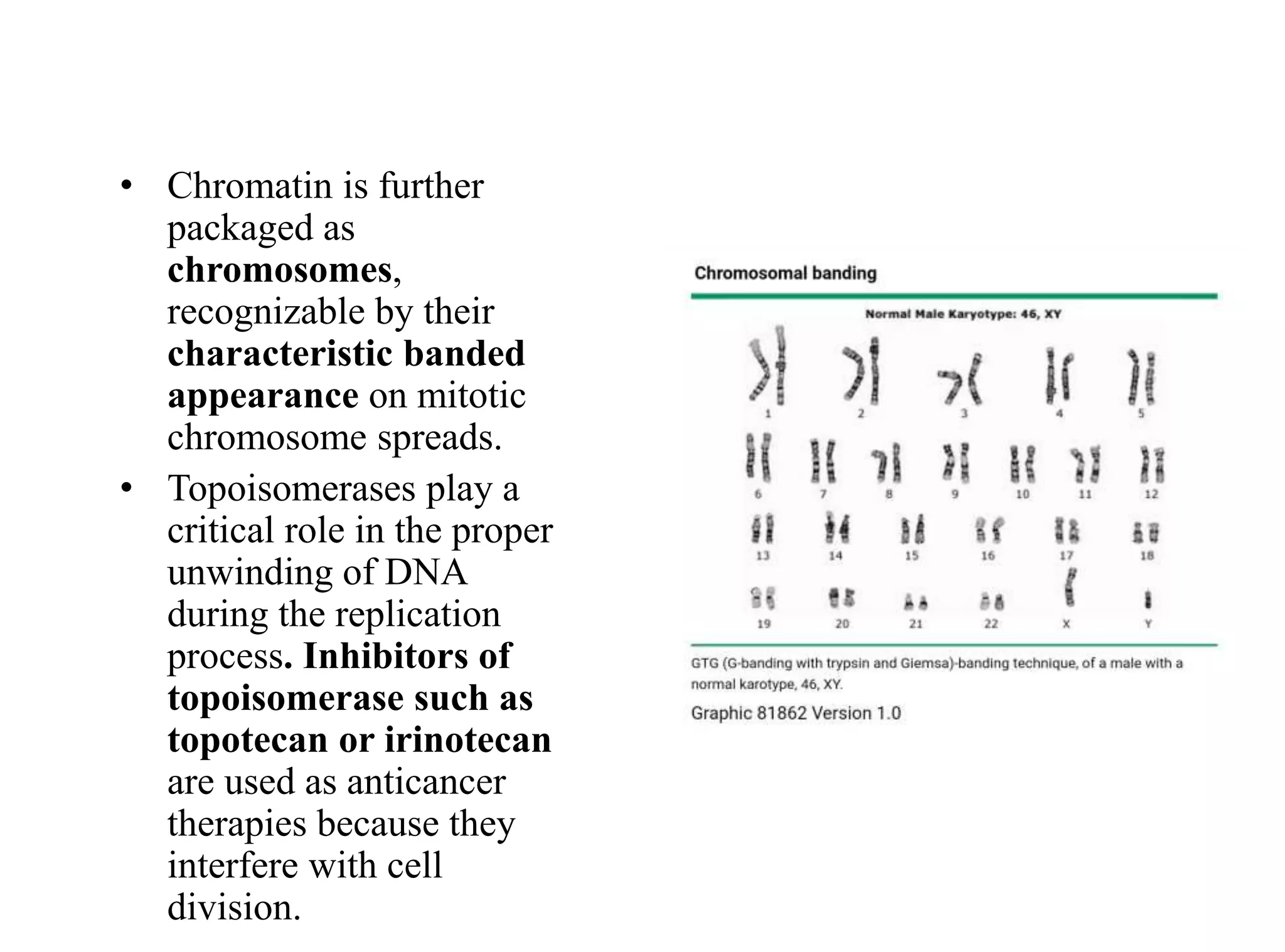
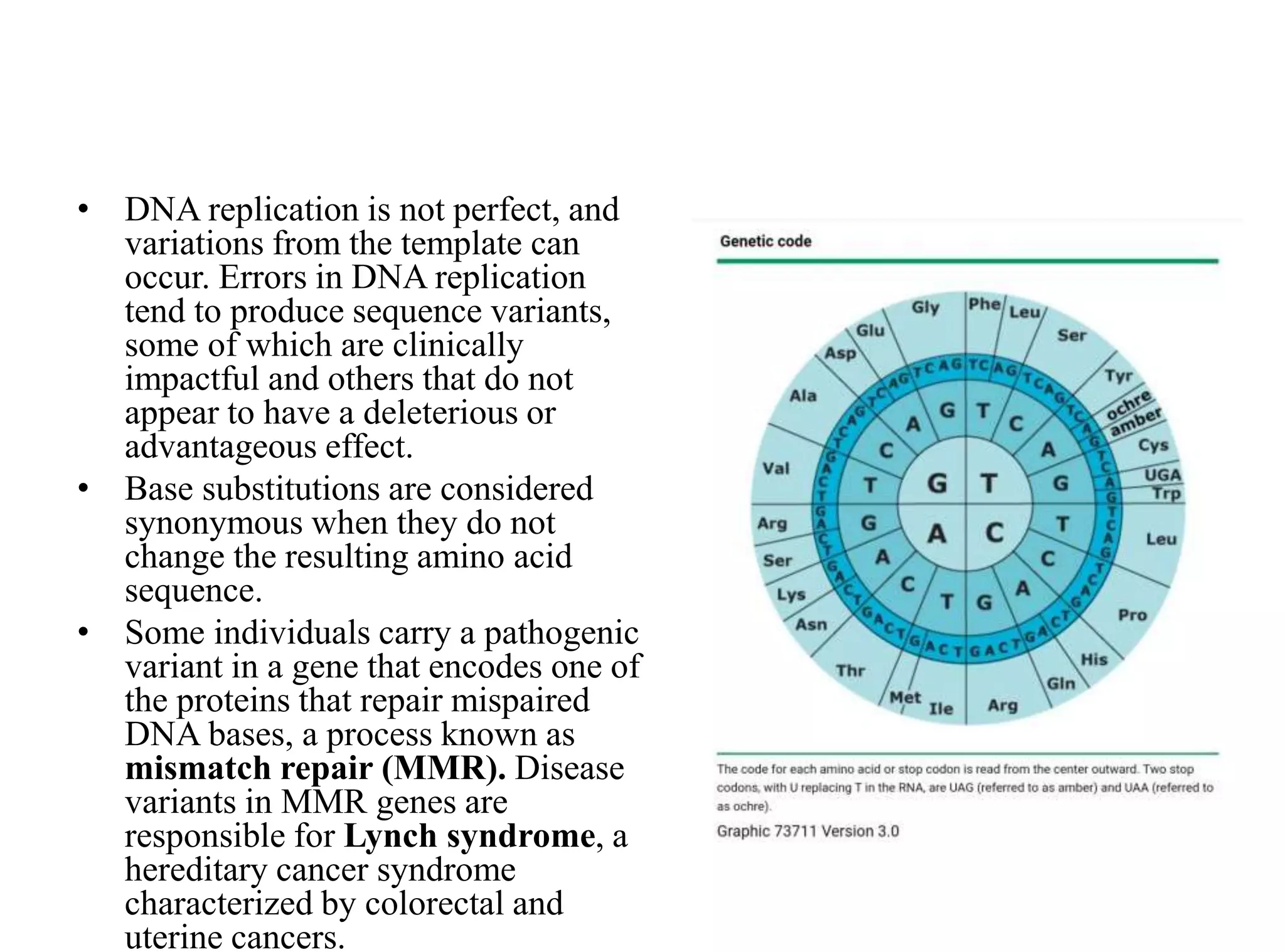
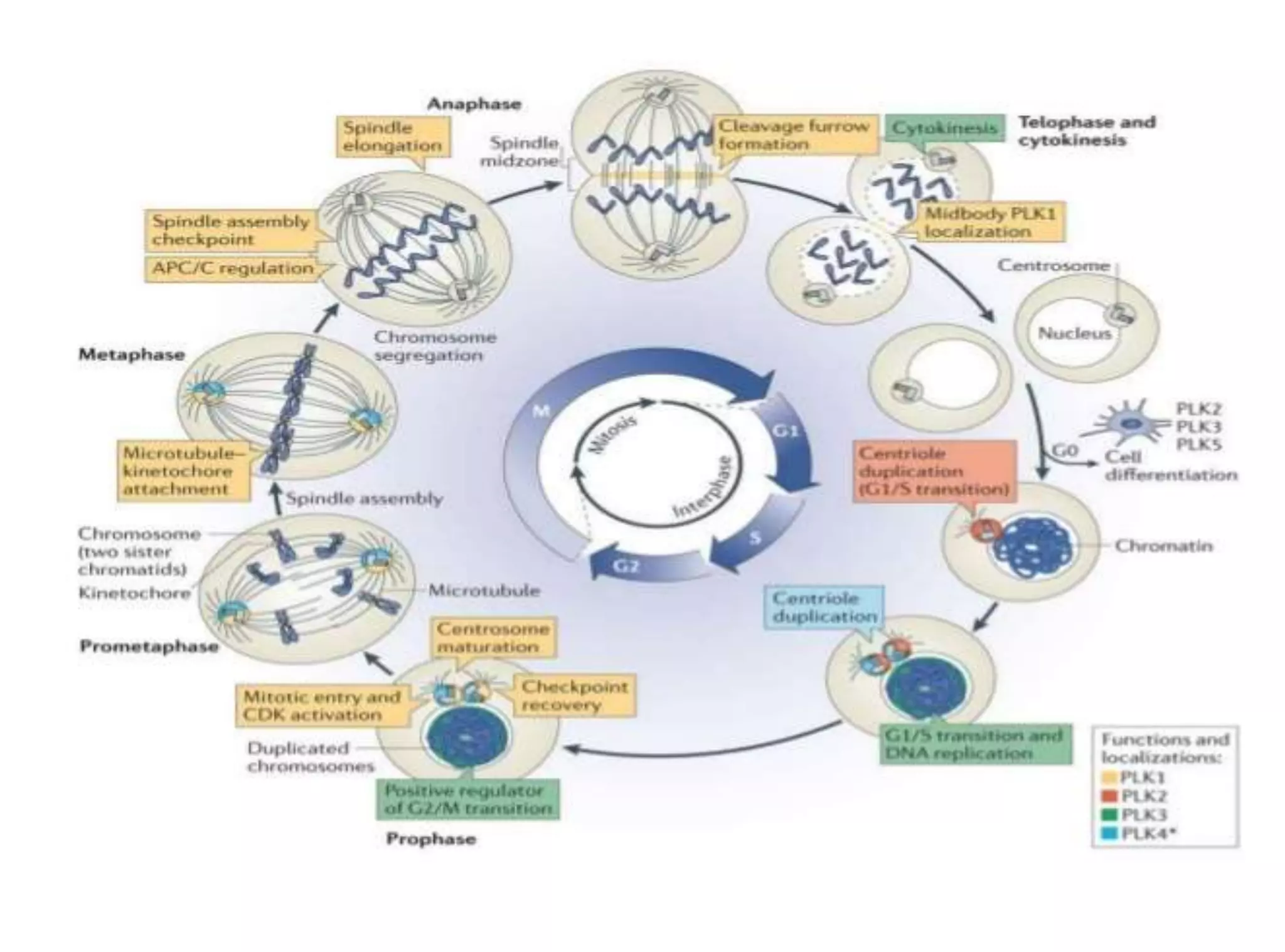
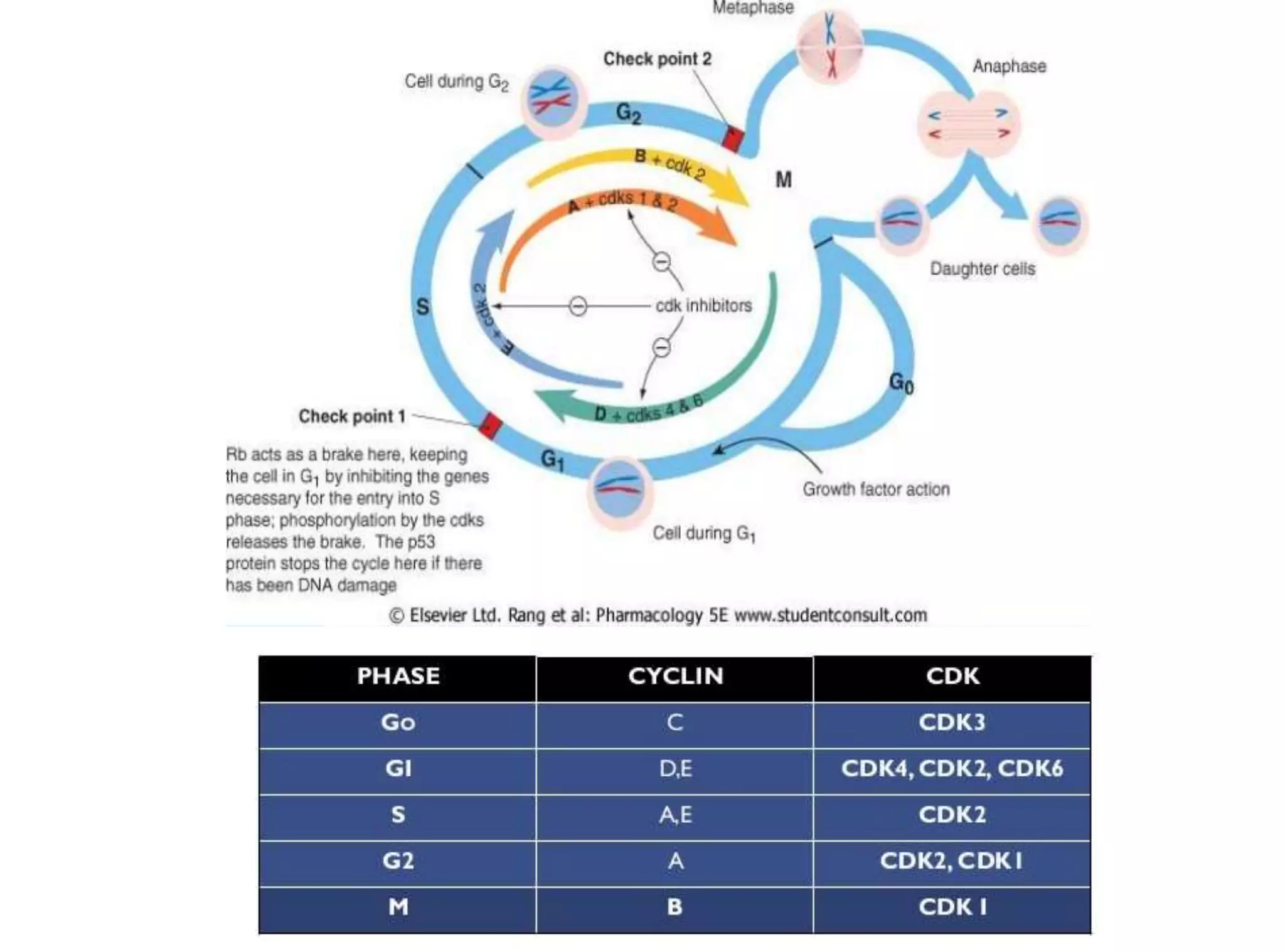
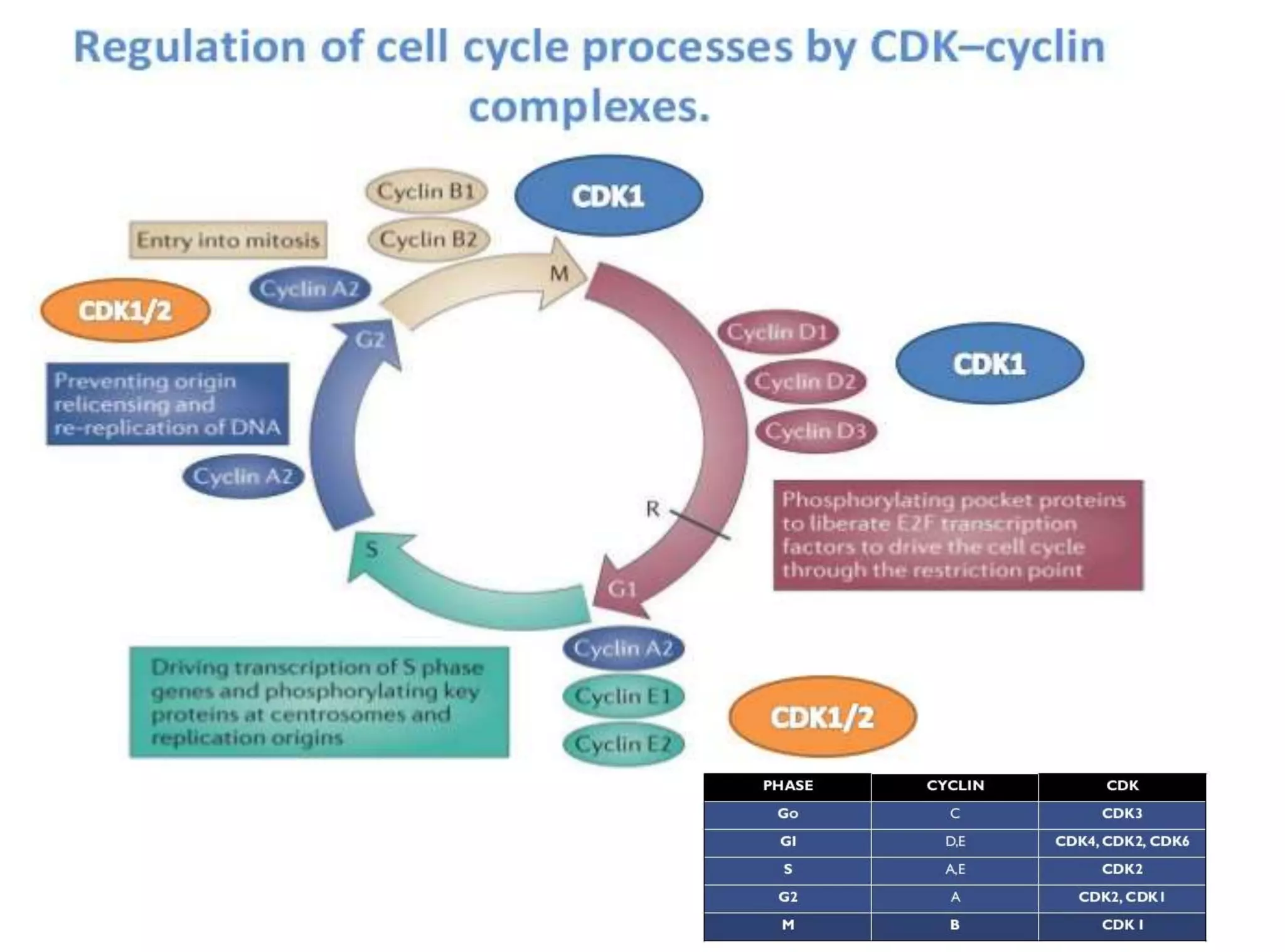
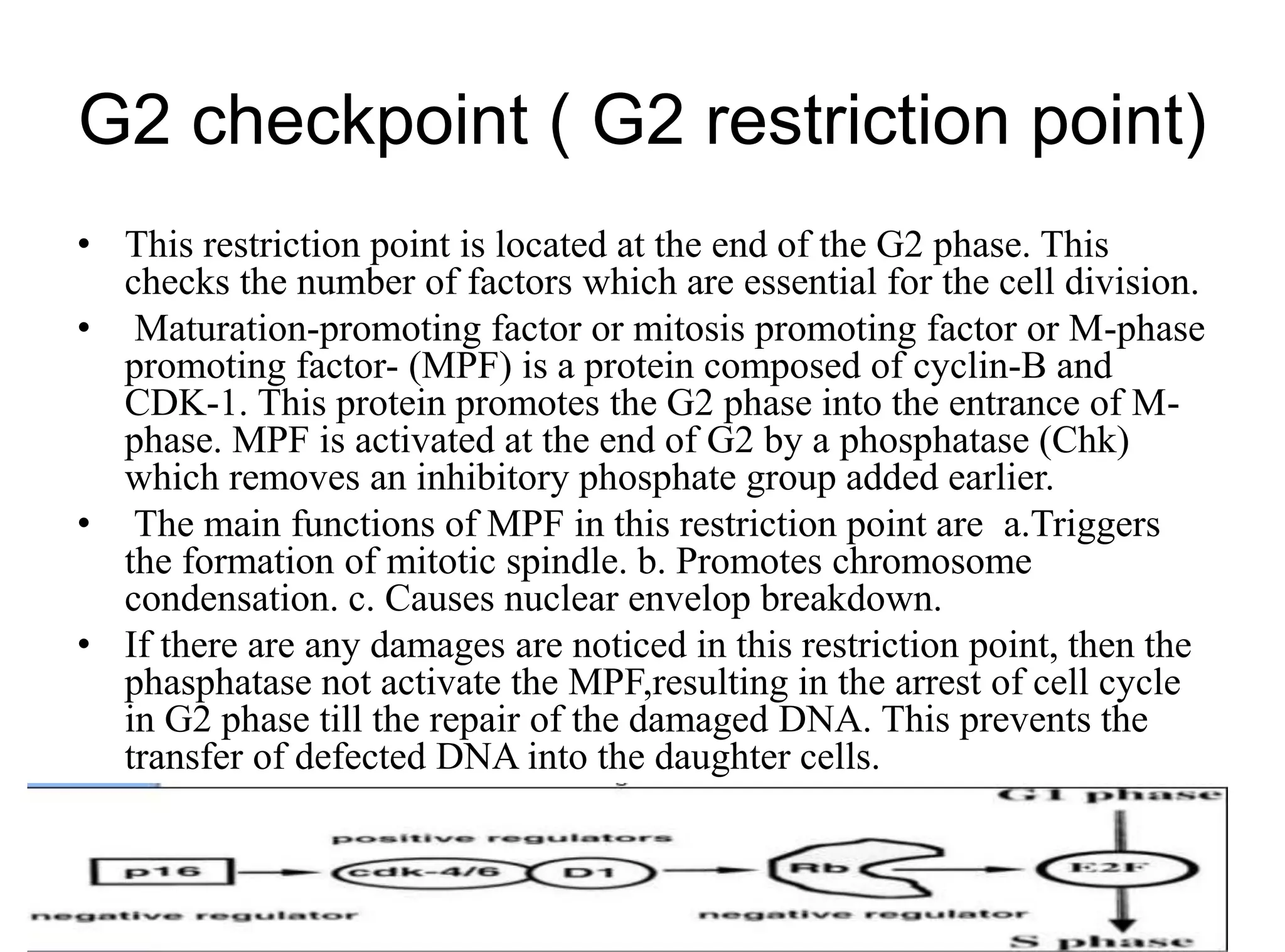
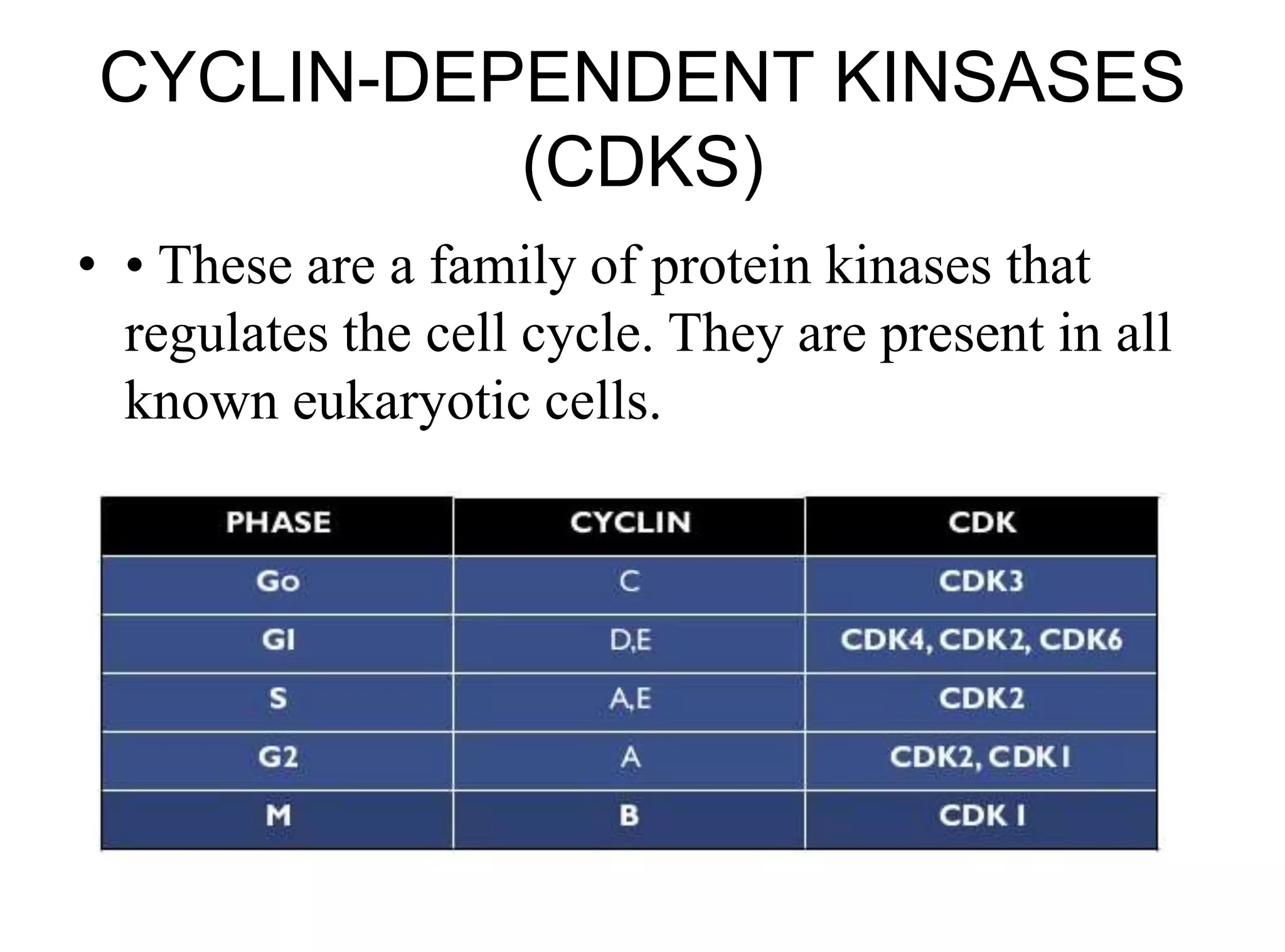
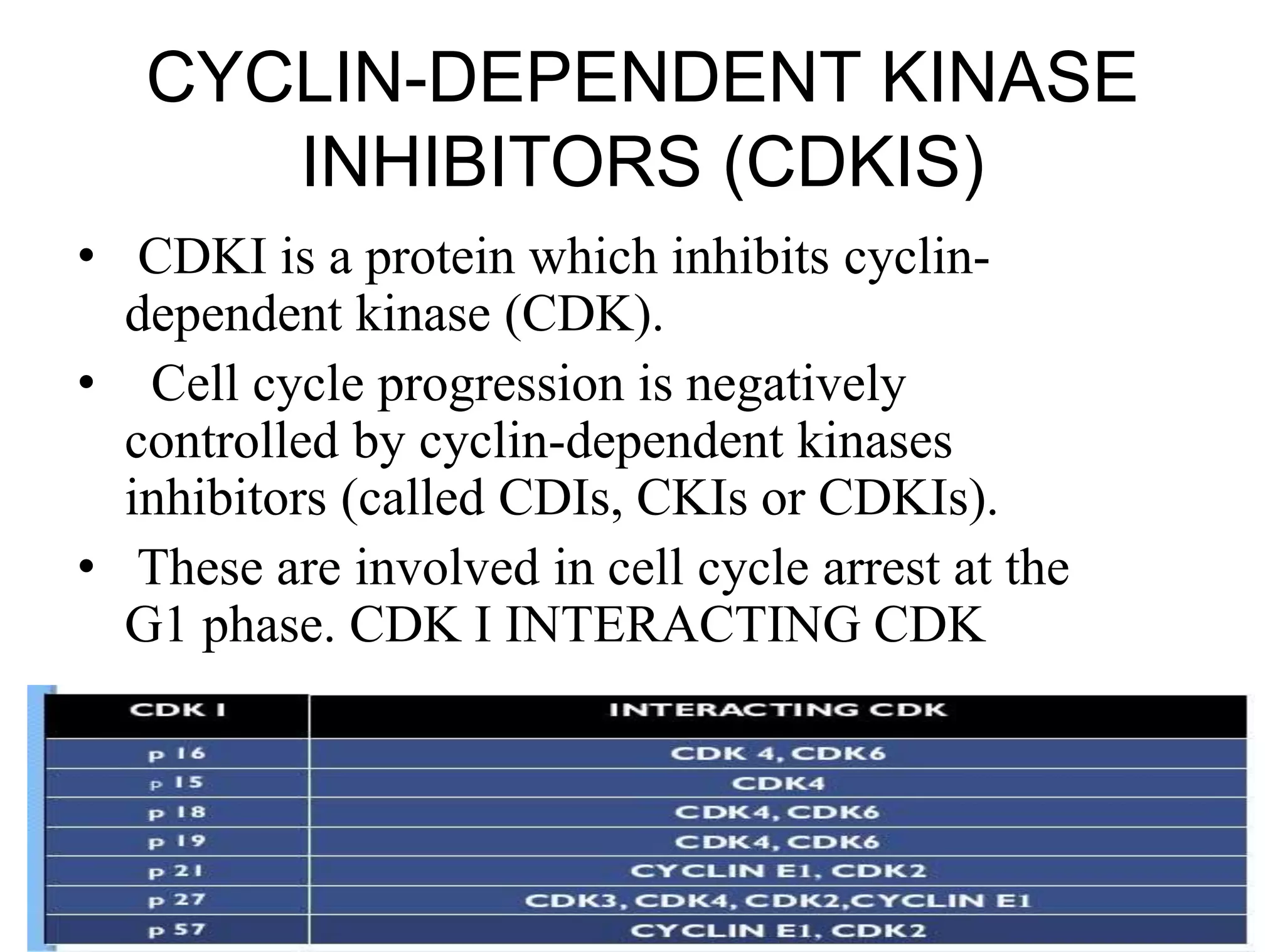
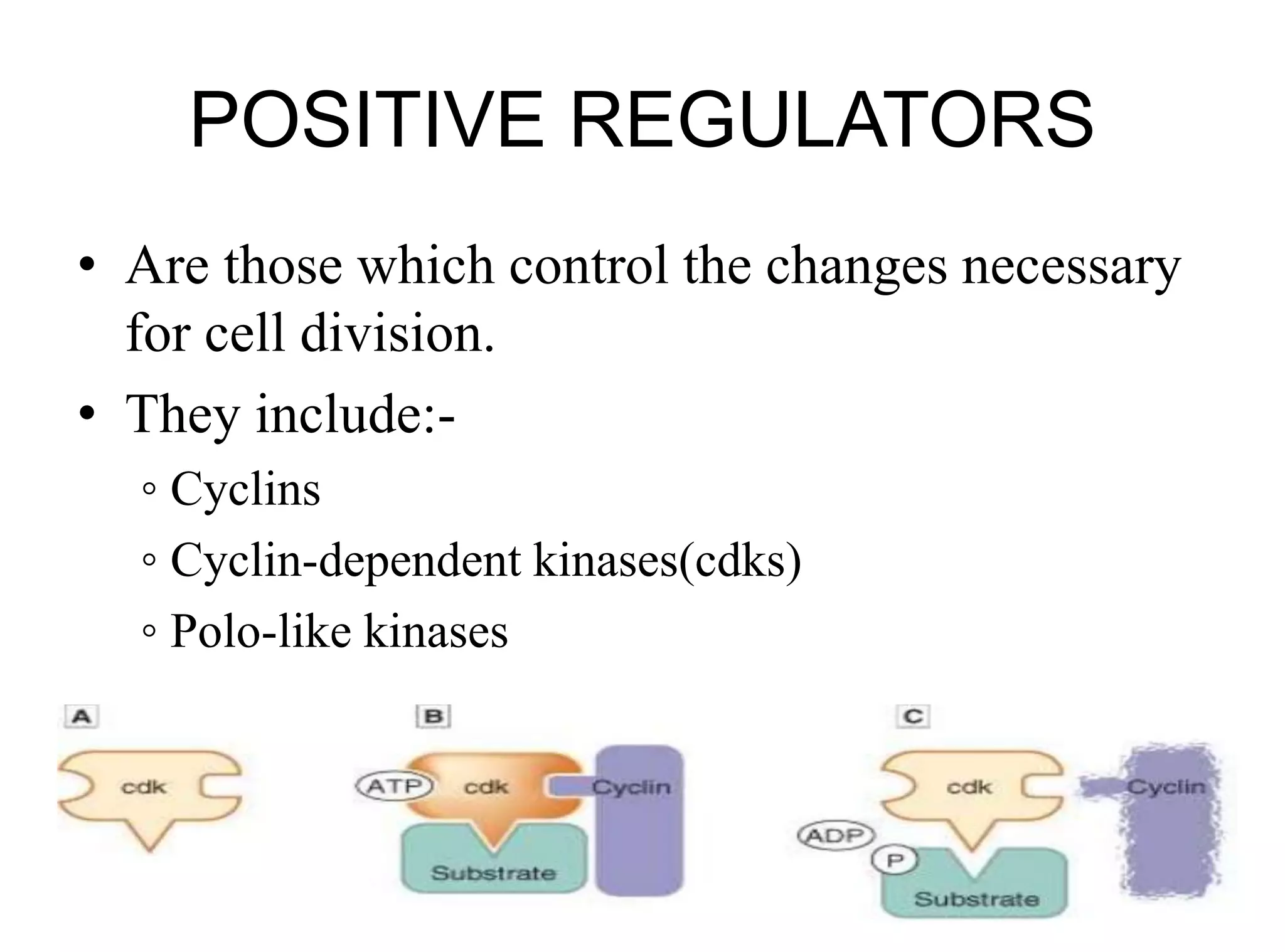
The document discusses chromosome organization and packaging, as well as the cell cycle and its regulation. It describes the different phases of the cell cycle (G1, S, G2, M) and checkpoints that ensure DNA replication is complete and any damage repaired before the cell divides. Key regulators of the cell cycle include cyclins, CDKs, and CDKIs, which promote or inhibit phase transitions in response to internal and external signals. DNA damage checkpoints in particular arrest the cell cycle to allow repair or induce apoptosis if damage is too severe.