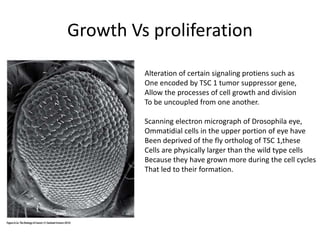
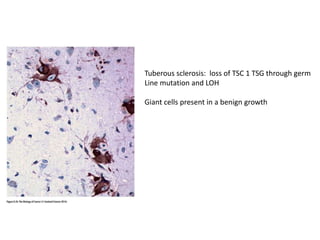
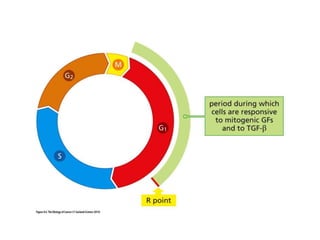
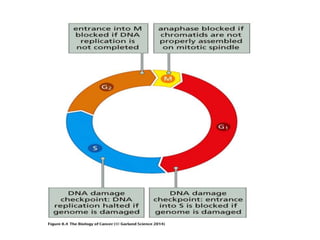
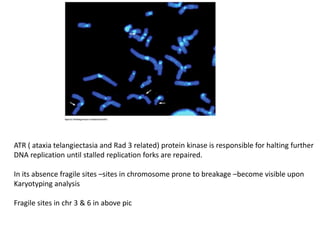
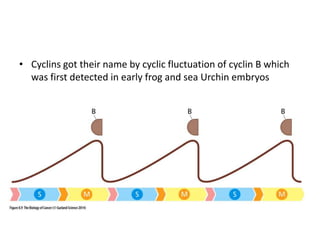
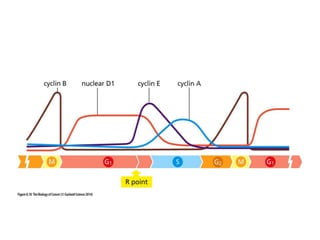
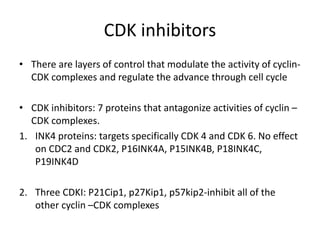
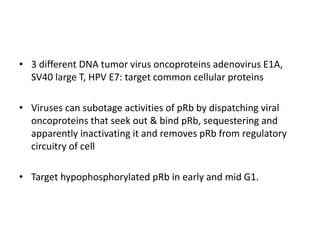
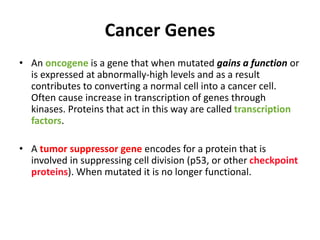
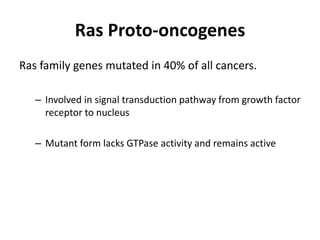
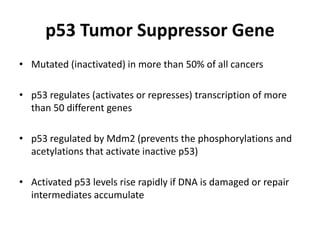
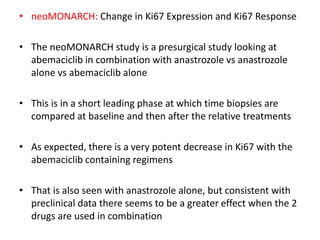
The document summarizes key aspects of the cell cycle and its implications for cancer therapy. It describes the cell cycle clock and checkpoints that regulate progression through the different phases. Dysregulation of cyclins, CDKs, and CDK inhibitors can disrupt normal cell cycle control and lead to uncontrolled proliferation. Tumor suppressor genes and oncogenes play important roles in cancer by influencing the cell cycle. Chemotherapy and radiation therapy target rapidly dividing cancer cells, aiming to push them through checkpoints where they are most vulnerable. CDK4 inhibitors show promise for breast cancer treatment by decreasing the proliferation marker Ki67.