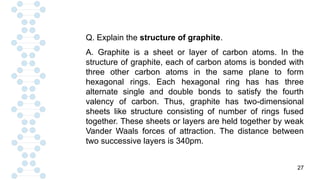
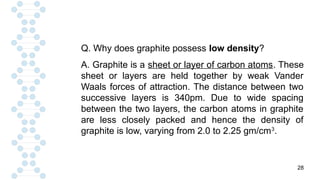
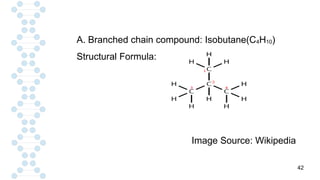
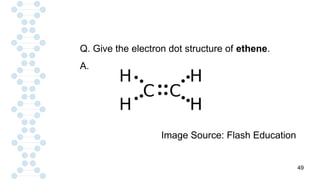
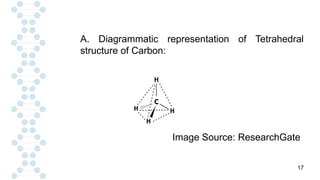
CARBON IS TETRAVALENT, MEANING IT HAS FOUR ELECTRONS IN IT'S SECOND SHELL. CARBON FORMS COVALENT BONDS WITH OTHER ELEMENTS. CARBON HAS A UNIQUE PROPERTY CALLED CATENATION, WHICH ALLOW IT TO FORM LONG CHAINS AND RINGS. CARBON IS ABUNDANT IN THE UNIVERSE, INCLUDING IN THE SUN, PLANETS, AND EARTH'S ATMOSPHERE.
![1
CLASS 10 SCIENCE
CARBON
AND
ITS COMPOUNDS
[ CHAPTER:1]](https://image.slidesharecdn.com/carbon-250220043655-8b9e92b9/75/CARBON-AND-IT-S-COMPOUNDS-CHAPTER-1-CLASS-10-SCIENCE-1-2048.jpg)

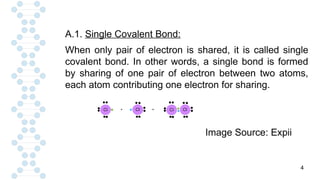
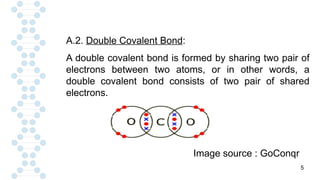
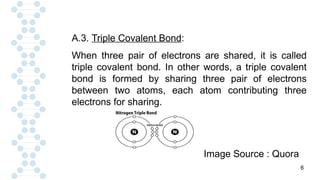






![19
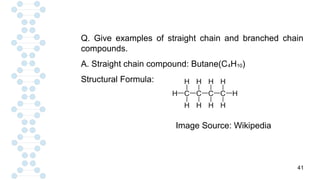
Q. Define catenation. What is the result of this property?
A. The existence of number of organic compounds is due
to the self-linking of carbon atoms which makes it
versatile in nature. The process of self-linking is called
catenation.
C – C – C – C – C
[Self-linking of carbon atoms]
As a result, the catenation property of carbon makes it
versatile in nature.The catenation results in the formation
of long chains of carbon atoms which may be branched
or straight in nature.](https://image.slidesharecdn.com/carbon-250220043655-8b9e92b9/85/CARBON-AND-IT-S-COMPOUNDS-CHAPTER-1-CLASS-10-SCIENCE-19-320.jpg)