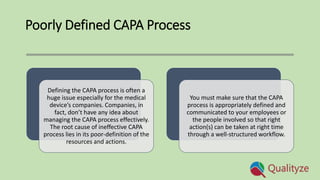
The document discusses common challenges in CAPA (Corrective and Preventive Action) management within regulated industries like life sciences, emphasizing the importance of effective processes, workflows, and technology. Key issues include lack of cross-functionality, reactive approaches, improper usage of CAPA, poor root cause identification, and poorly defined processes, each impacting the effectiveness of quality management. Solutions proposed involve adopting comprehensive CAPA software, establishing cross-functional teams for reviews, and ensuring clear communication of processes to improve overall quality management systems.