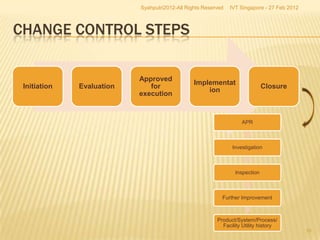
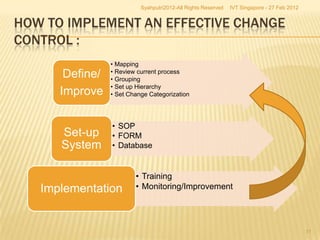
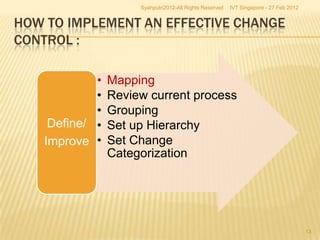
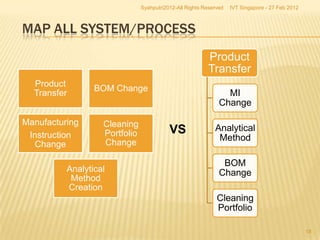
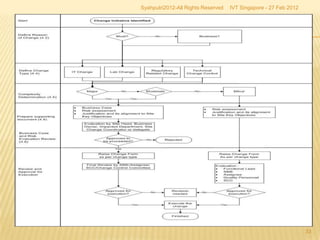
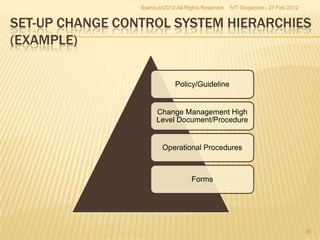
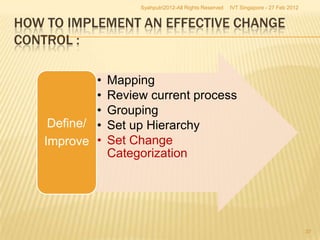
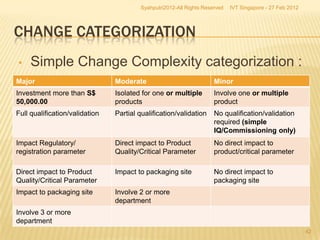
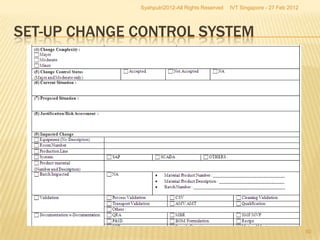
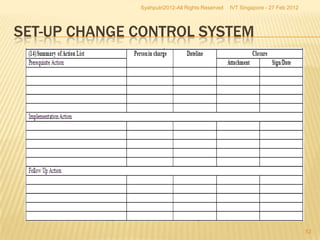
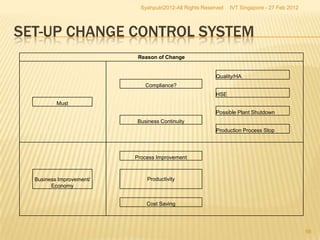
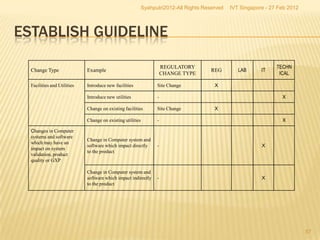
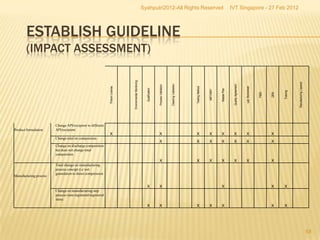
The document outlines best practices for implementing an effective change control program in a company, emphasizing the importance of a systematic approach to managing changes in products or systems. It details regulatory requirements, procedures for maintaining change control, and the necessity for thorough documentation and assessment of changes to ensure quality and compliance. The document also highlights the role of quality management and the various steps necessary for mapping, reviewing, and categorizing changes in a structured manner.