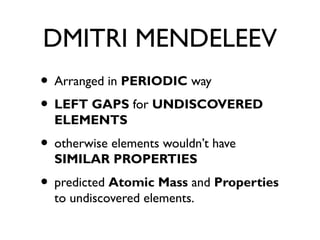
The document discusses the history and development of the periodic table. It describes early periodic tables from the 1800s with fewer than 40 known elements arranged based on atomic mass. John Newlands proposed the law of octaves but it only worked for the first few elements. Dmitri Mendeleev arranged elements in a periodic way with gaps for undiscovered elements and was able to predict properties. The modern periodic table arranges elements by atomic number and groups them based on electron configuration in the outer shell leading to similar properties within groups. It also discusses trends in reactivity down groups and across periods.








![GROUP 2
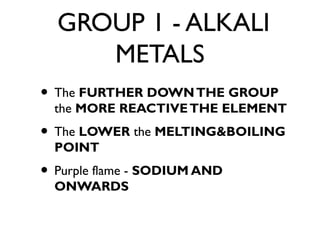
TRANSITION METALS
• Build buildings due to being LESS
REACTIVE
• Form IONS with different charges
• Form COLOURED compounds
[COPPER SULFATE - BLUE]
• Useful as CATALYSTS](https://image.slidesharecdn.com/c3revision-140313150425-phpapp01/85/C3-revision-Chemistry-unit-3-13-320.jpg)
![GROUP 7
HALOGENS
• Bad conductors
• POISONOUS
• Coloured vapours
• React with METALS to form IONIC
COMPOUNDS [ SODIUM CHLORIDE]](https://image.slidesharecdn.com/c3revision-140313150425-phpapp01/85/C3-revision-Chemistry-unit-3-14-320.jpg)




![TRENDS IN
REACTIVITY
• GROUP 1 elements need to LOSE
electrons to react.
• LESS ENERGY LEVELS:
• STRONGER attraction [ To NUCLEUS]
• Less SCREENING/SHIELDING by
INNER ELECTRONS
• Electrons lost LESS easily](https://image.slidesharecdn.com/c3revision-140313150425-phpapp01/85/C3-revision-Chemistry-unit-3-19-320.jpg)
![TRENDS IN
REACTIVITY
• MORE ENERGY LEVELS:
• Outer Electron FURTHER from nucleus
• WEAKER attraction [ TO NUCLEUS]
• MORE screening/Shielding by inner
electrons
• Electron lost EASILY](https://image.slidesharecdn.com/c3revision-140313150425-phpapp01/85/C3-revision-Chemistry-unit-3-20-320.jpg)
![GROUP 7
REACTIVITY
• Needs to GAIN electron
• LESS energy levels :
• Outer electron CLOSER to nucleus
• Stronger attraction [ TO NUCLEUS]
• LESS screening/shielding by INNER
ELECTRONS](https://image.slidesharecdn.com/c3revision-140313150425-phpapp01/85/C3-revision-Chemistry-unit-3-21-320.jpg)
![GROUP 7
REACTIVITY
• MORE energy levels :
• Outer electron FURTHER to nucleus
• Weaker attraction [ TO NUCLEUS]
• MORE screening/shielding by INNER
ELECTRONS](https://image.slidesharecdn.com/c3revision-140313150425-phpapp01/85/C3-revision-Chemistry-unit-3-22-320.jpg)

![INTRO
• SOFT water : can LATHER [produce
bubbles when soaped]
• HARD water : CAN’T Lather : creates
SCUM&SCALE
• contains DISSOLVED COMPOUNDS
• AND MAGNESIUM&CALCIUM
compounds [river flows over substances]](https://image.slidesharecdn.com/c3revision-140313150425-phpapp01/85/C3-revision-Chemistry-unit-3-24-320.jpg)
![HARD WATER
• SCUM : INSOLUBLE precipitate
• Formed by REACTION with SOAP
• CA2+ [Calcium Ion] REACTS with
HARD WATER
• MORE soap needed [more expensive]
• SOAPLESS detergents made to avoid
scum [no Sodium Stearate won’t react]](https://image.slidesharecdn.com/c3revision-140313150425-phpapp01/85/C3-revision-Chemistry-unit-3-25-320.jpg)


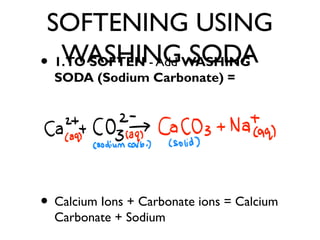
![SOFTENING HARD
WATER
• Contains CA2+ [CALCIUM IONS]
• Contains MG2+ [MAGNESIUM IONS]
• TO SOFTEN - Add WASHING SODA
(Sodium Carbonate) =](https://image.slidesharecdn.com/c3revision-140313150425-phpapp01/85/C3-revision-Chemistry-unit-3-28-320.jpg)






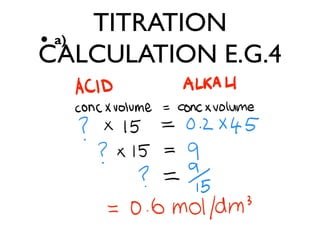
![TITRATION
CALCULATION
• 1 decimetre cubed = 1000cm³ = 1 LITRE
• CONC. OF SOLUTION = Moles per
dm³ [ 3 moles per 1 dm³ ]
• MOLES - mass [g] of 1 MOLE of a
substance is it’s RELATIVE FORMULA
MASS in Grams. [ Add all relative atomic
masses in a compound together = 1 mole]](https://image.slidesharecdn.com/c3revision-140313150425-phpapp01/85/C3-revision-Chemistry-unit-3-36-320.jpg)
![TITRATION
CALCULATION E.G.
• Make 1 mole per dm³ solution of Sodium
Hydroxide NaOH
• Relative atomic masses =
• Na=23 O=16 H=1 1 MOLE = 40g
• Take 1dm³ [1000 cm³ ] of water and put
40g of NaOH in it](https://image.slidesharecdn.com/c3revision-140313150425-phpapp01/85/C3-revision-Chemistry-unit-3-37-320.jpg)