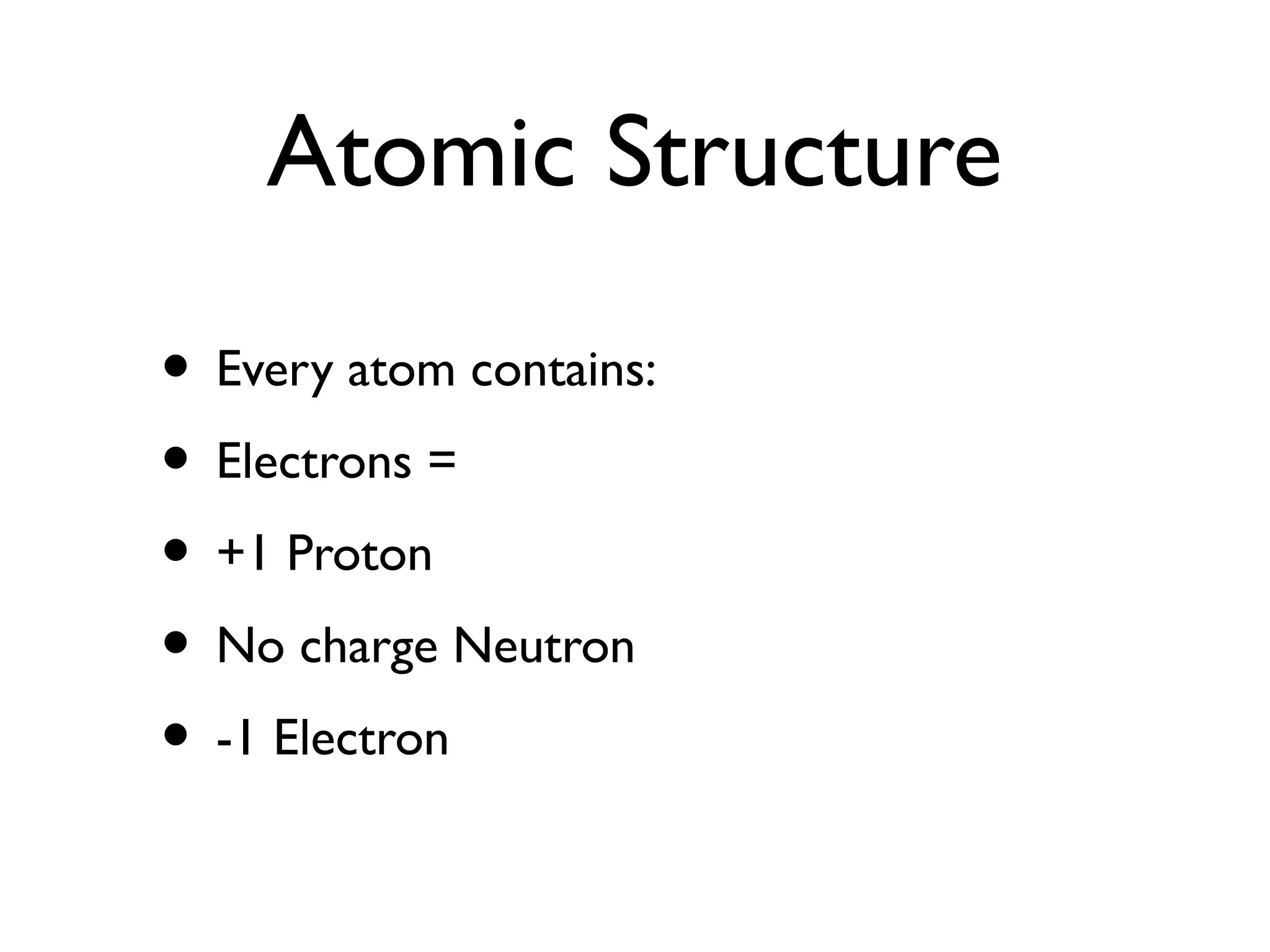
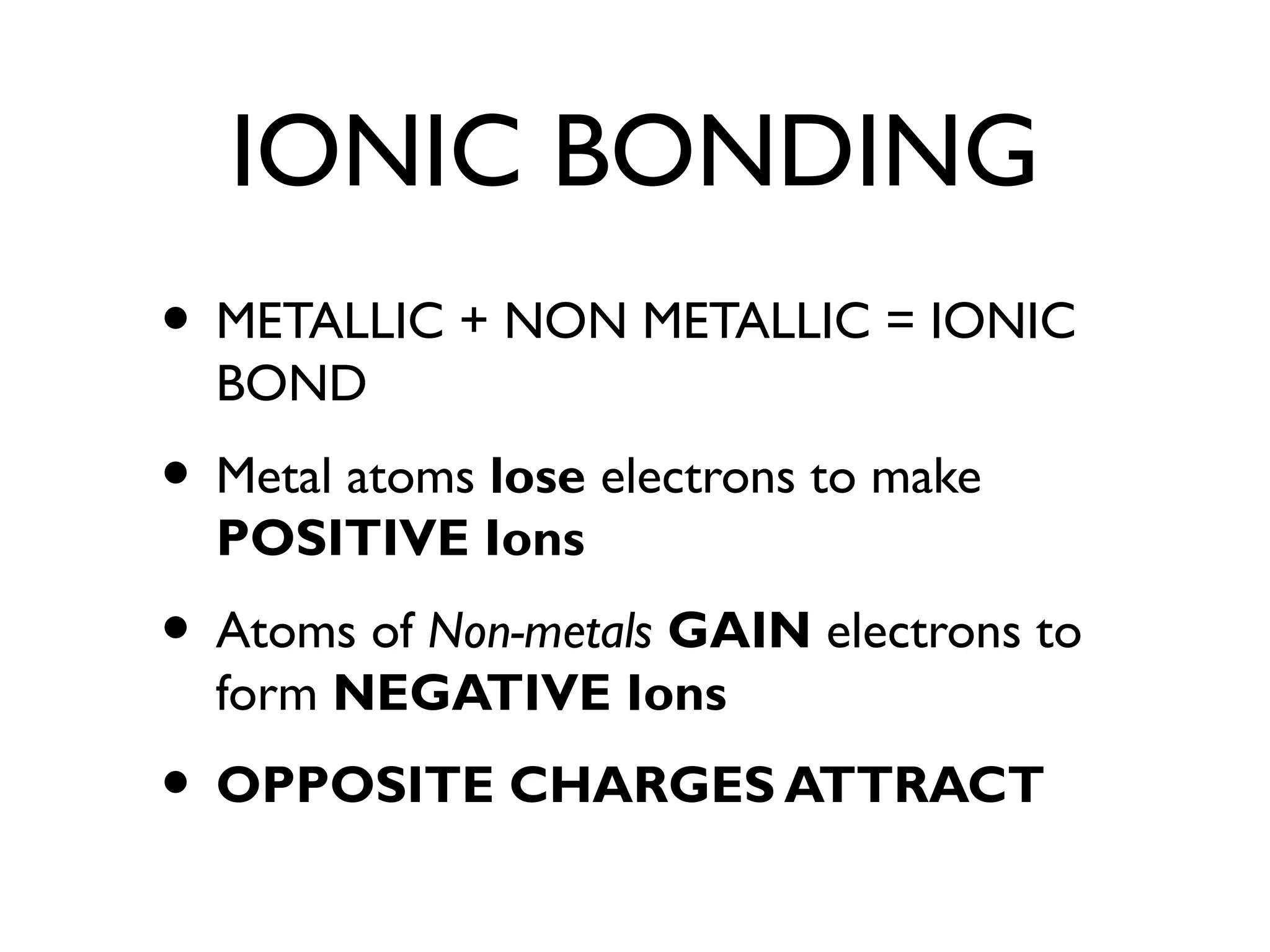
The document discusses atomic structure, ionic and covalent bonding, limestone and its uses in construction, extracting metals, crude oil and its fractional distillation, polymers, emulsions, and saturated and unsaturated fats. It explains that atoms contain protons, neutrons and electrons, and how ionic and covalent bonding occurs. It also describes how limestone is used to make cement, mortar and concrete, and the limestone cycle.





![LIMESTONE AND
BUILDING MATERIAL
• USED FOR :
• Building STATUES
• Limestone + Clay = CEMENT
• Limestone + Clay +Sand = MORTAR
• Limestone + Clay + Sand + Aggregate
[Small stones] = CONCRETE](https://image.slidesharecdn.com/c1revision-140313150204-phpapp01/75/C1-revision-Chemistry-unit-1-8-2048.jpg)

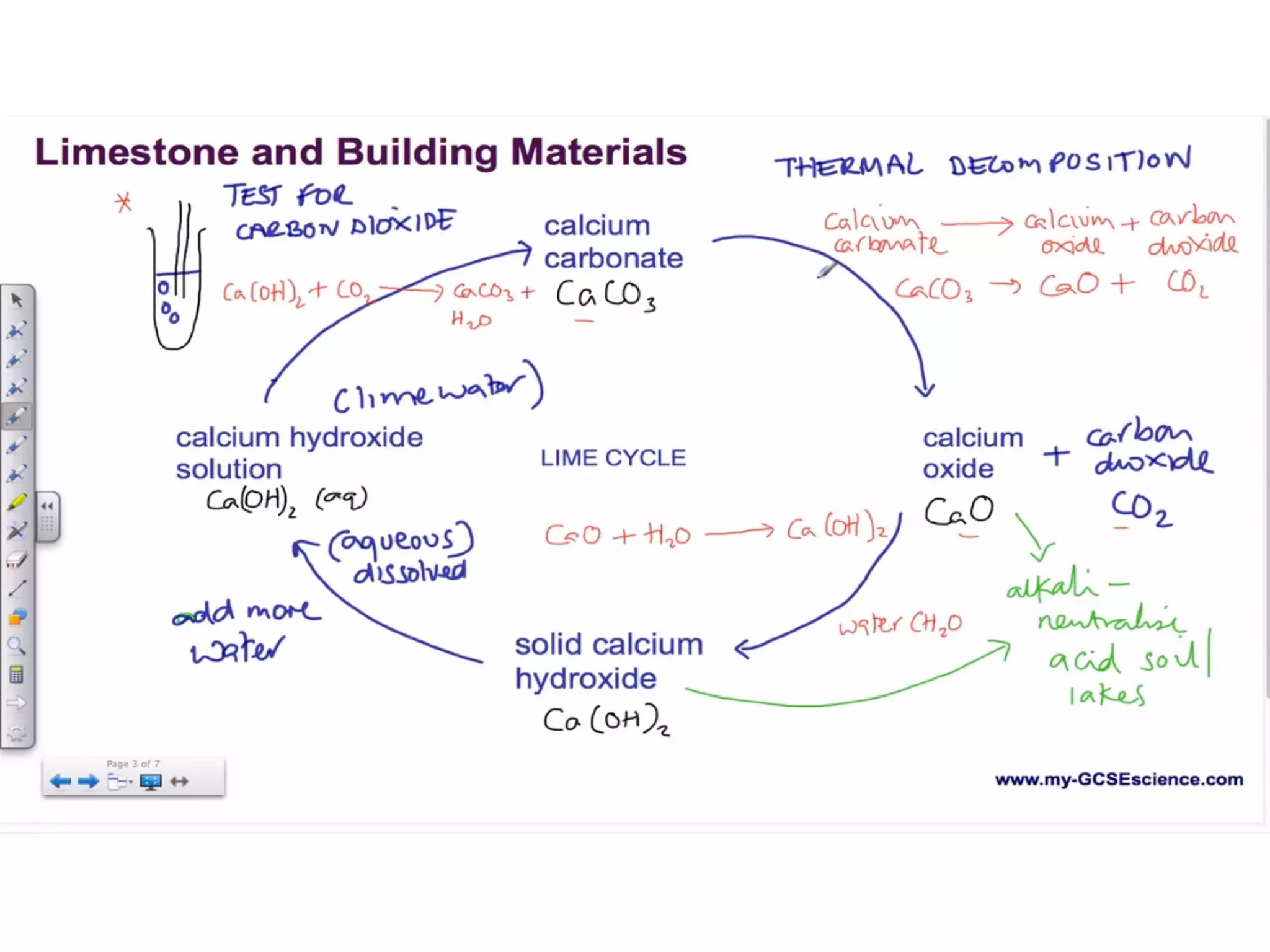
![LIMESTONE CYCLE
• Calcium Carbonate[CaCo3] THERMAL
DECOMPOSITION
• Calcium Oxide [CaO]---> Solid Calcium
Hydroxide [Ca(OH)2] --> Calcium
Hydroxide Solution [Ca(OH)2 (aq)]](https://image.slidesharecdn.com/c1revision-140313150204-phpapp01/75/C1-revision-Chemistry-unit-1-10-2048.jpg)










![HOW TO SEPARATE
CRUDE OIL MIXTURE
• IDEA :
• Boiling tube has 3 LIQUID
COMPOUNDS
• EACH has it’s own BOILING POINT
e.g. 70º 150º and 350º [ Temp liquid>Gas]
• Boil to evaporate liquids until ONE IS
LEFT](https://image.slidesharecdn.com/c1revision-140313150204-phpapp01/75/C1-revision-Chemistry-unit-1-22-2048.jpg)
![DISTILLATION
[separating liquids]
• LOW Boiling point :
• HIGHLY FLAMMABLE
• Good for FUEL
• LOW viscosity [ THICKNESS ]](https://image.slidesharecdn.com/c1revision-140313150204-phpapp01/75/C1-revision-Chemistry-unit-1-23-2048.jpg)
![DISTILLATION
[separating liquids]
• HIGH Boiling point :
• LOW FLAMMABILITY
• HIGH viscosity [ THICKNESS ]](https://image.slidesharecdn.com/c1revision-140313150204-phpapp01/75/C1-revision-Chemistry-unit-1-24-2048.jpg)



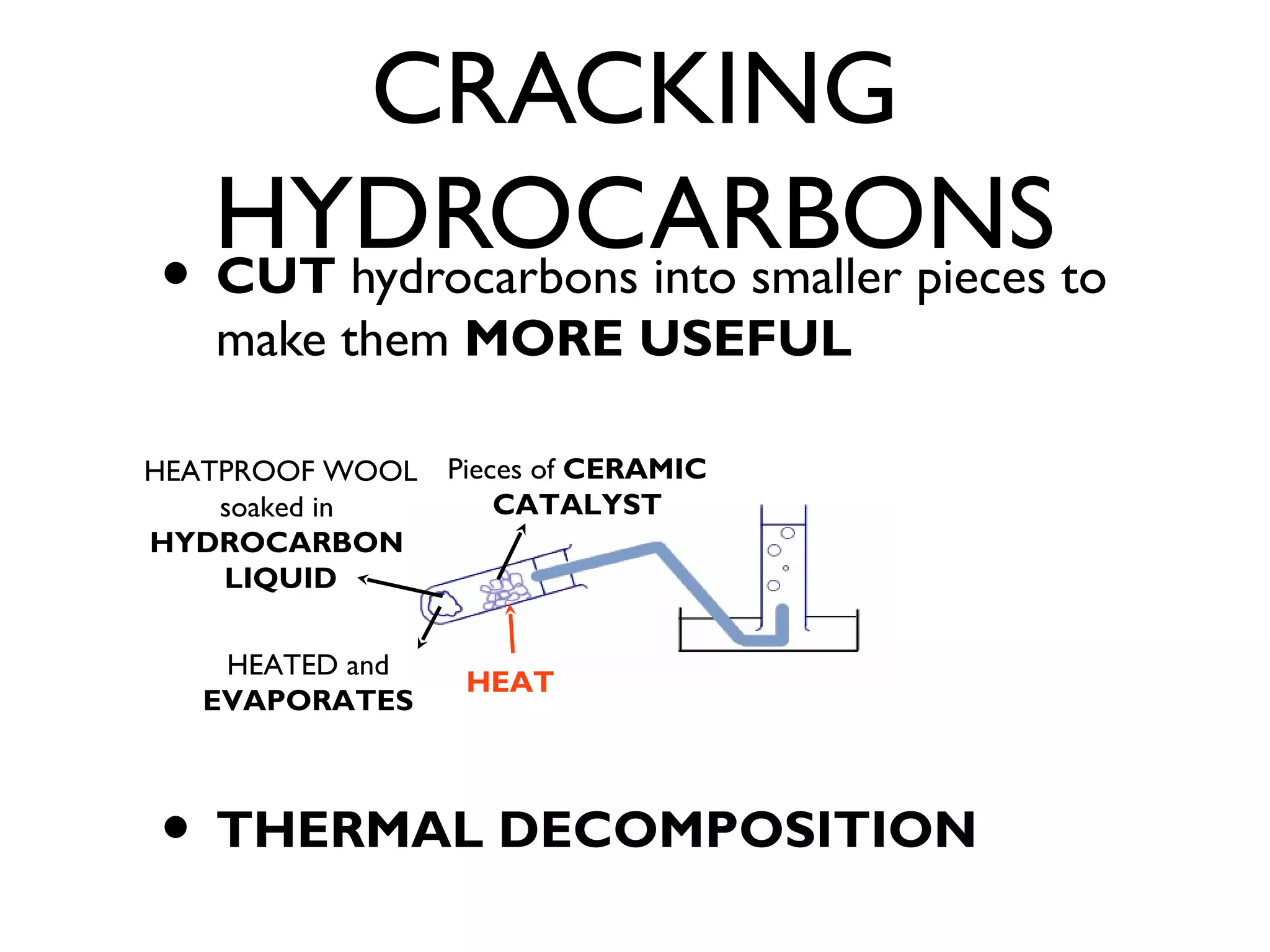
![CRACKING
HYDROCARBONS
• ALKANE becomes [NO double bonds]
• -->
• Shorter Hydrocarbon Chain ; MORE
USEFUL because SHORT CHAIN [Has
ALL good features]](https://image.slidesharecdn.com/c1revision-140313150204-phpapp01/75/C1-revision-Chemistry-unit-1-29-2048.jpg)

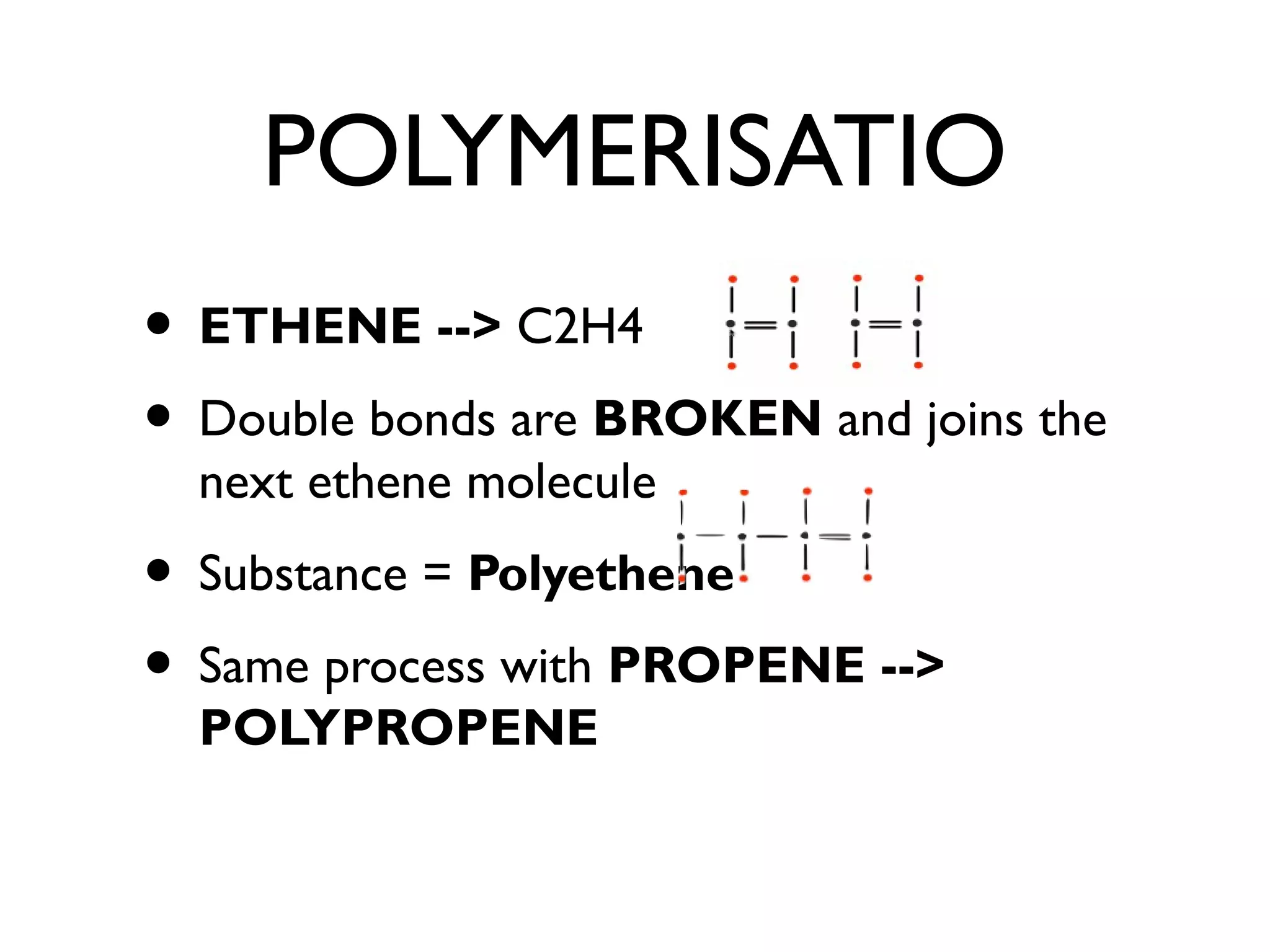
![POLYMERS
• ETHENE is an ALKENE =
FLAMMABLE
• LONG Chain of Ethene - React it and it
makes a POLYMER [polymerisation]
• Products of Ethene GAS is different to
POLYETHENE [plastic product]
• Molecules called MONOMERS](https://image.slidesharecdn.com/c1revision-140313150204-phpapp01/75/C1-revision-Chemistry-unit-1-31-2048.jpg)
![BIODEGRADABLE
POLYMERS
• In LANDFILL SITES after a few years,
POLYMERS will not rot --> VERY
UNREACTIVE
• To make them BIODEGRADABLE [Can
be EATEN by BACTERIA] you add
CORNSTARCH.](https://image.slidesharecdn.com/c1revision-140313150204-phpapp01/75/C1-revision-Chemistry-unit-1-33-2048.jpg)



![REACT ETHENE WITH
STEAM
• HYDRATION REACTION
• Ethene +Water [steam] --> ETHANOL](https://image.slidesharecdn.com/c1revision-140313150204-phpapp01/75/C1-revision-Chemistry-unit-1-37-2048.jpg)


![EMULSIONS ARE...
• ...MOREVISCOUS
• BETTER at COATING [sticky]
• Better TEXTURE
• e.g. Salad DRESSING ; Mayonnaise ; Ice
cream ; Milk ; Paint ; Cosmetics](https://image.slidesharecdn.com/c1revision-140313150204-phpapp01/75/C1-revision-Chemistry-unit-1-41-2048.jpg)


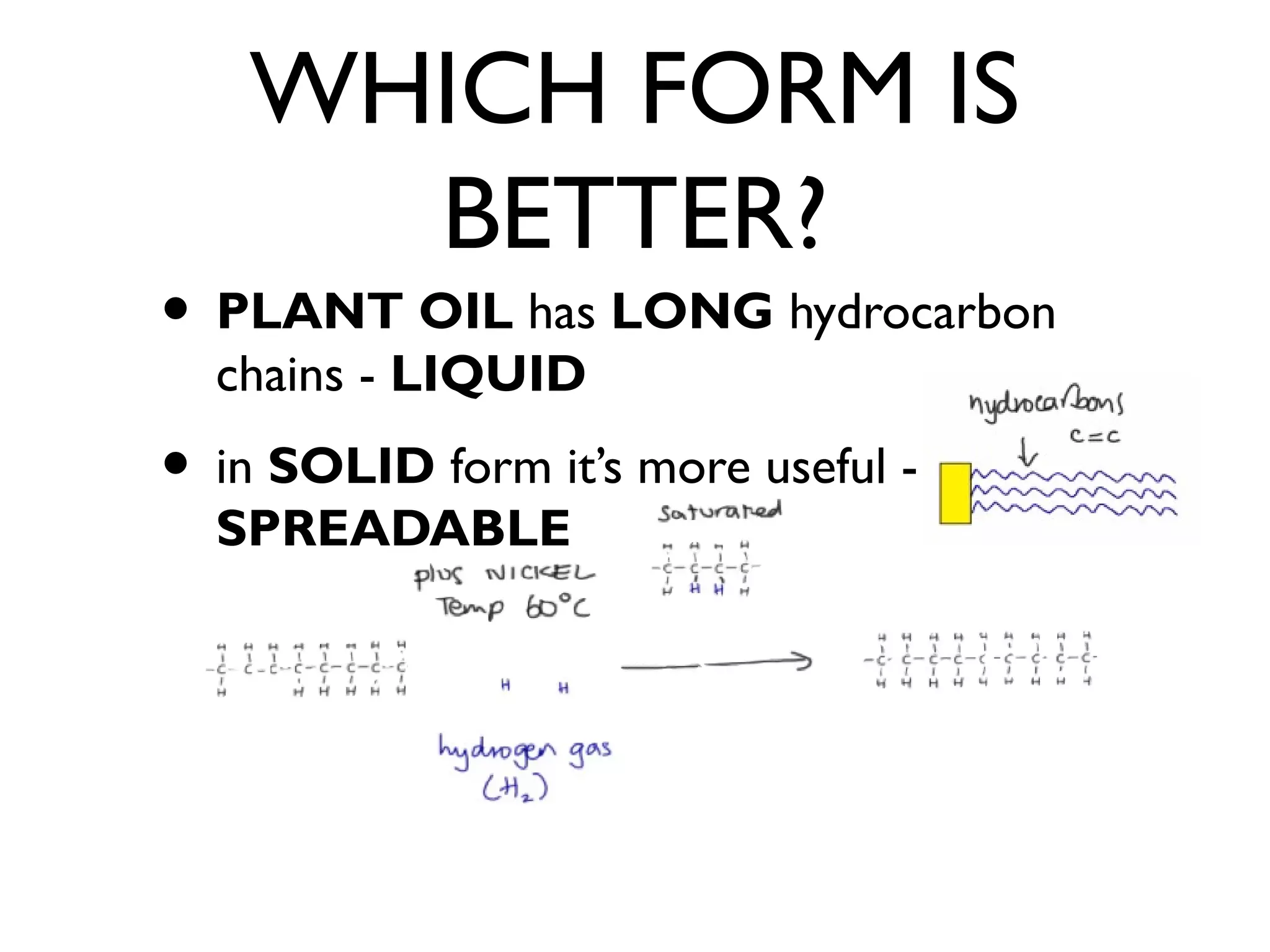
![SATURATED AND
UNSATURATED FATS
• SATURATED FATS
• SOLID [High MELTING point]
• Come from ANIMALS [butter]
• LESS healthy [+ BLOOD CHOLESTEROL]
• UNSATURATED FATS [ oil ]
• LIQUID [Low MP]
• PLANTS [Olive Oil]](https://image.slidesharecdn.com/c1revision-140313150204-phpapp01/75/C1-revision-Chemistry-unit-1-44-2048.jpg)
![SATURATED AND
UNSATURATED FATS
• SATURATED FATS
• NO DOUBLE BONDS
• UNSATURATED FATS [ oil ]](https://image.slidesharecdn.com/c1revision-140313150204-phpapp01/75/C1-revision-Chemistry-unit-1-45-2048.jpg)