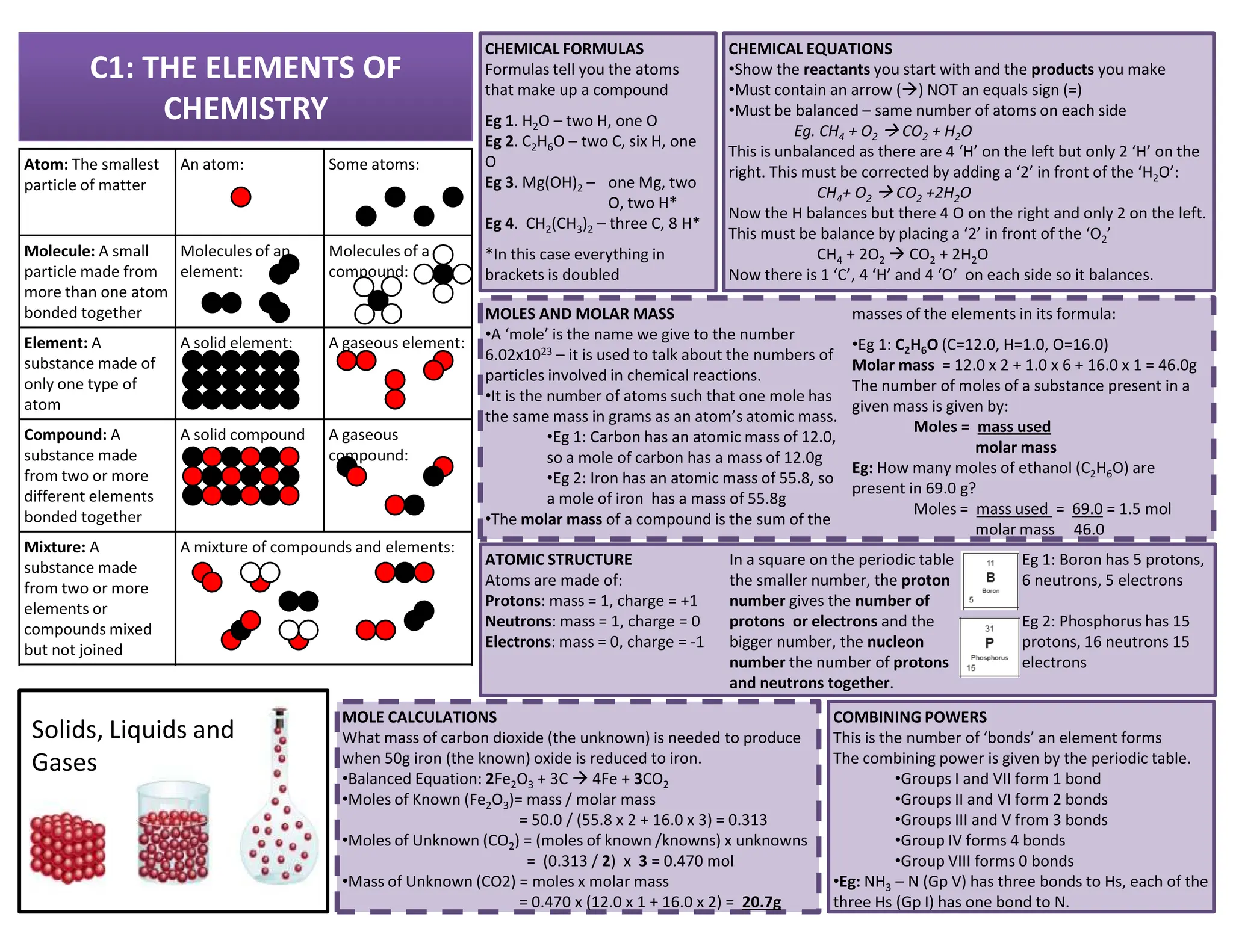
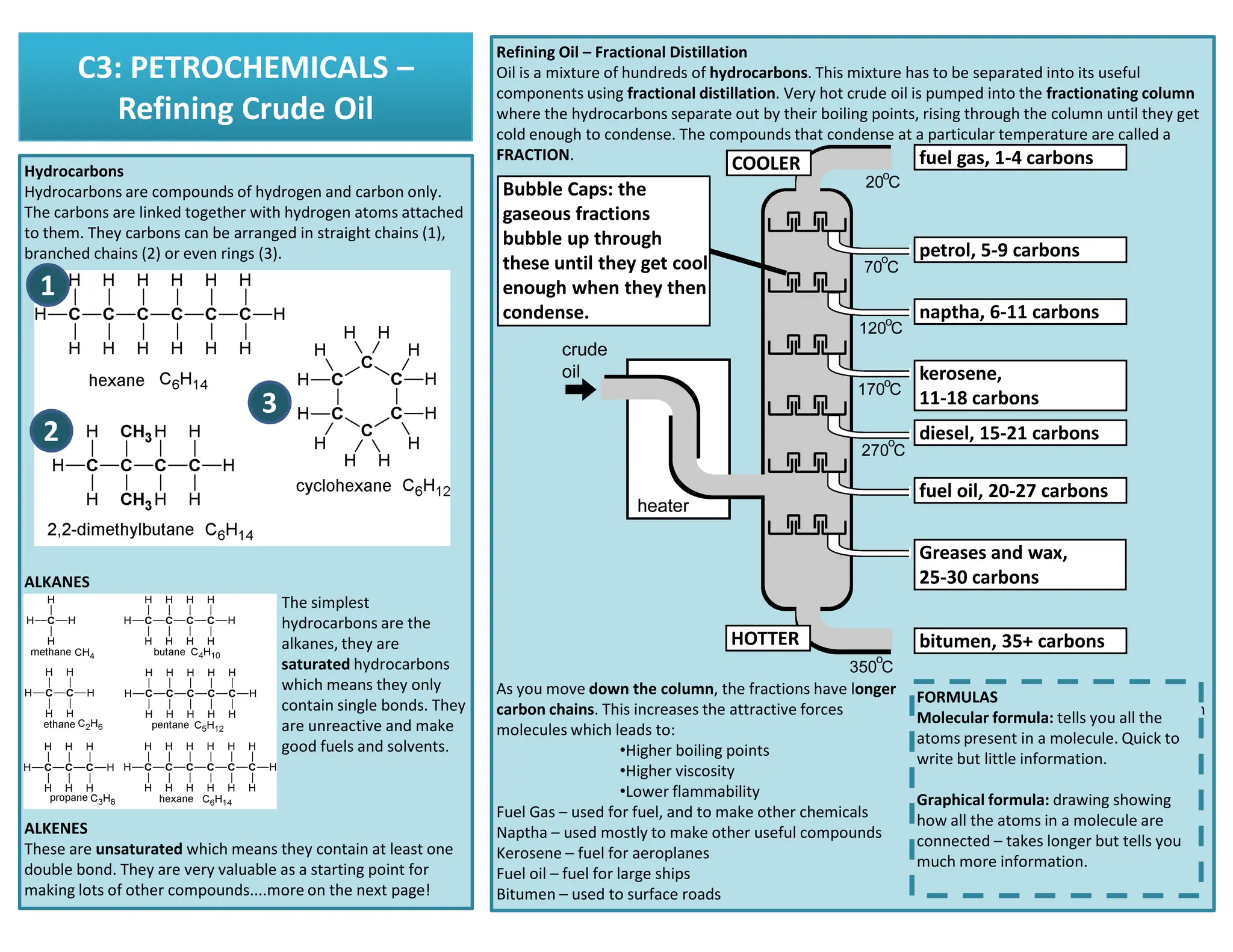
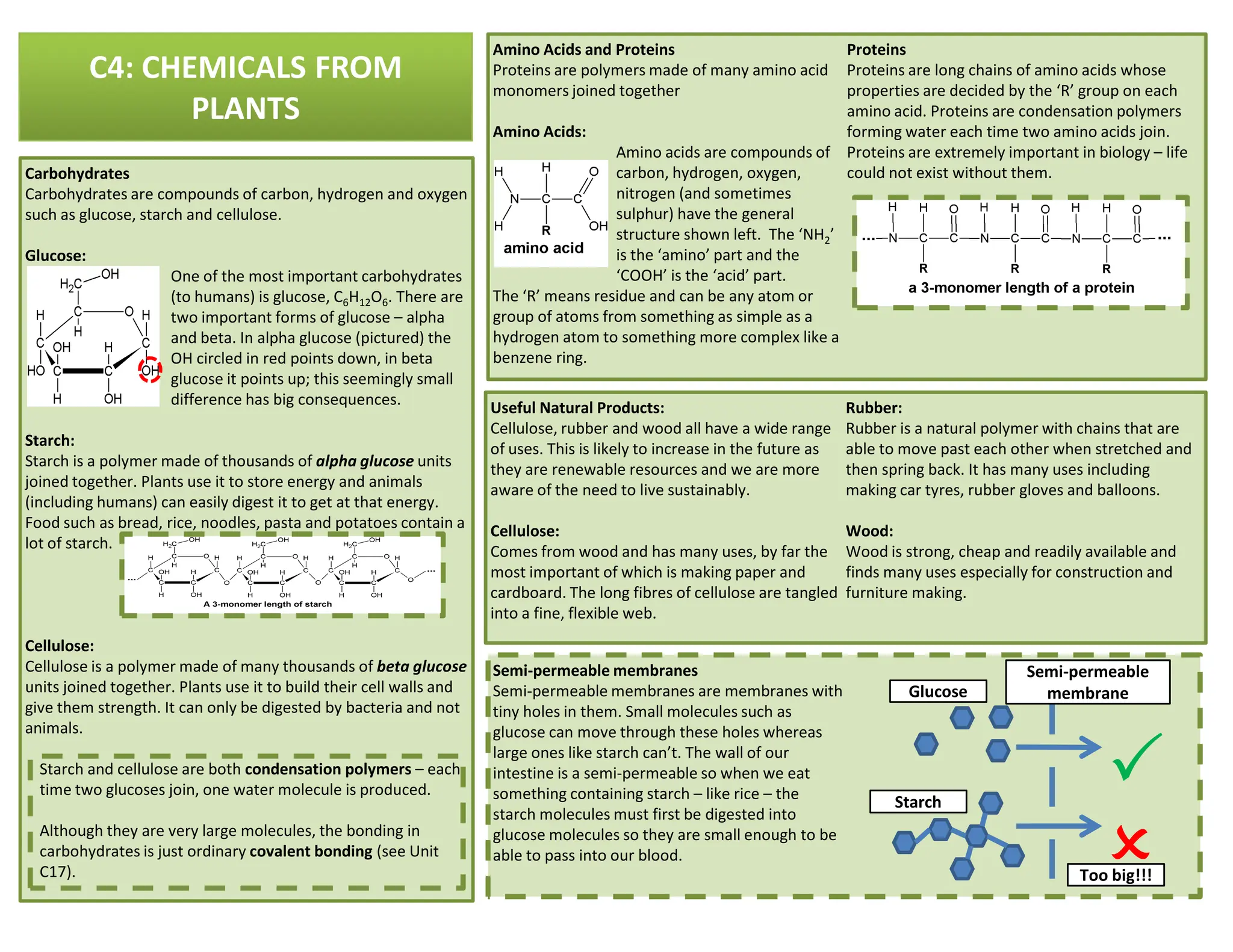
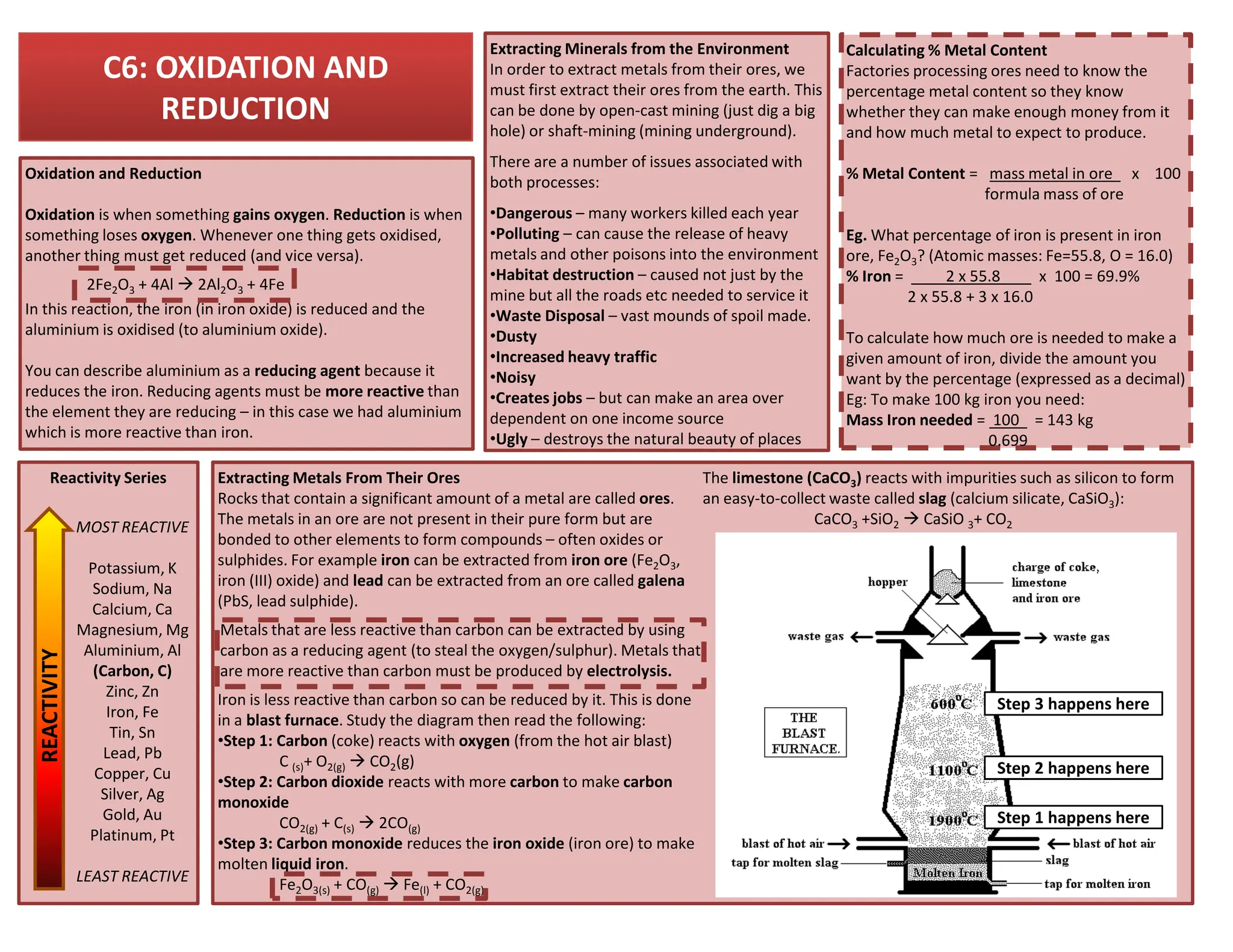
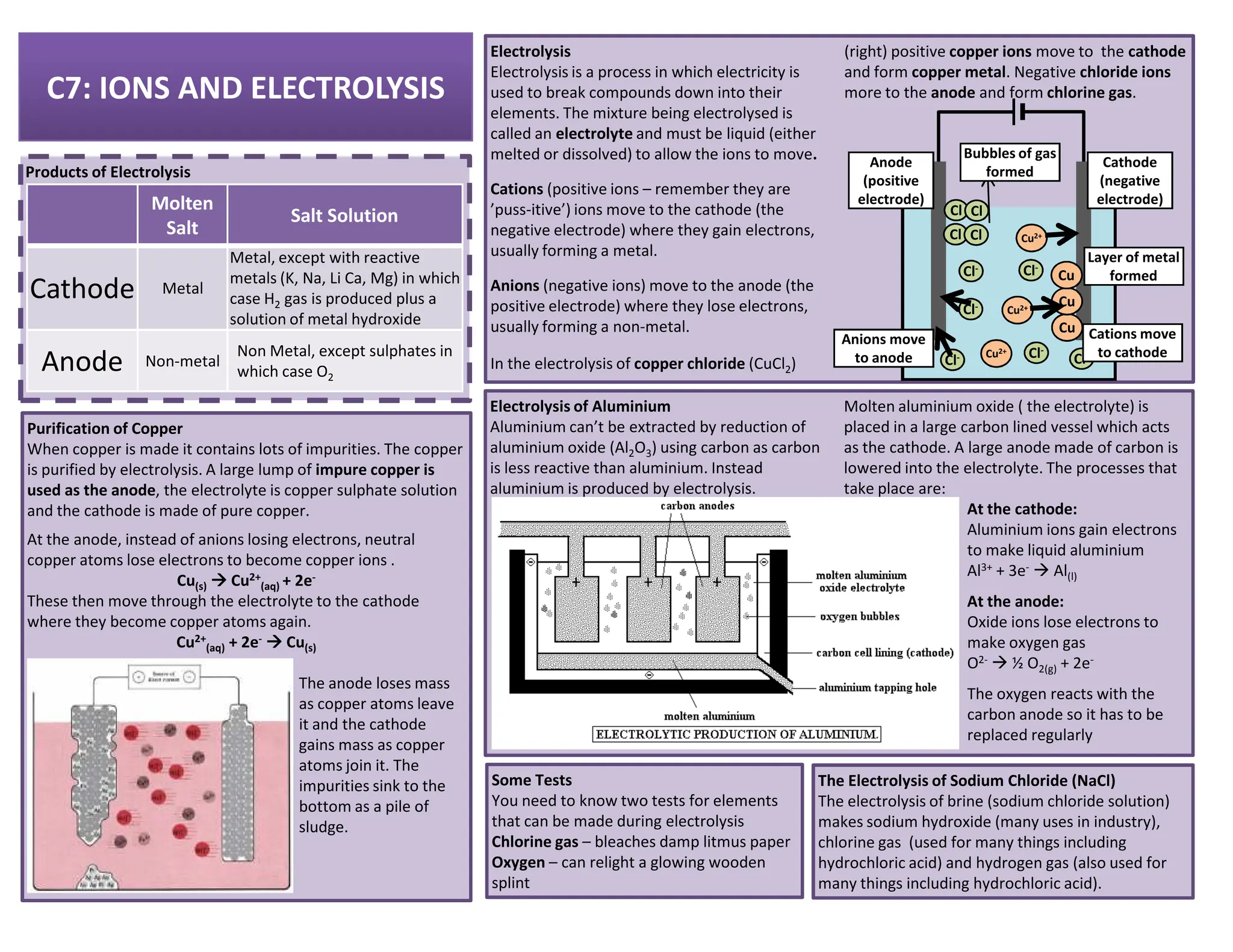
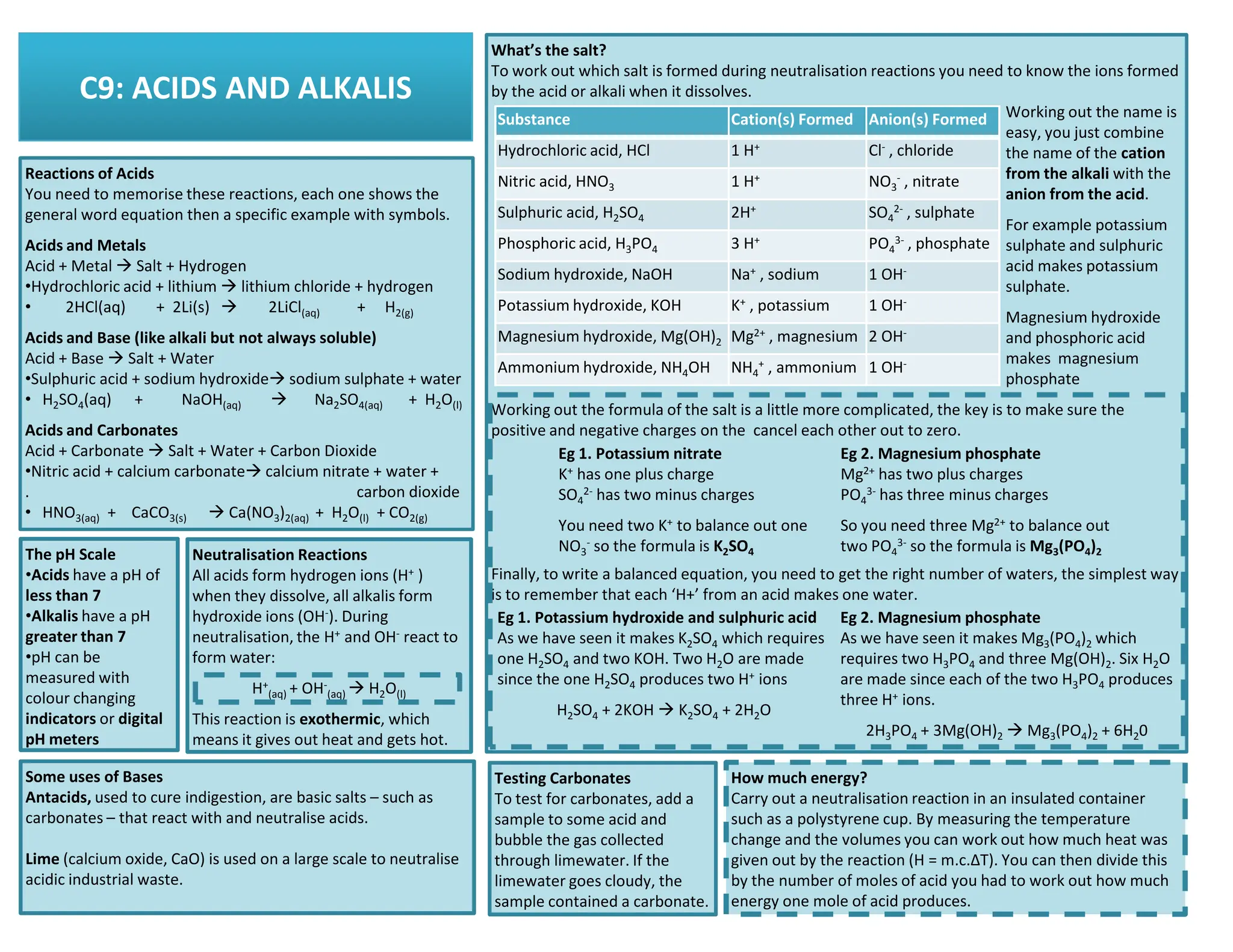
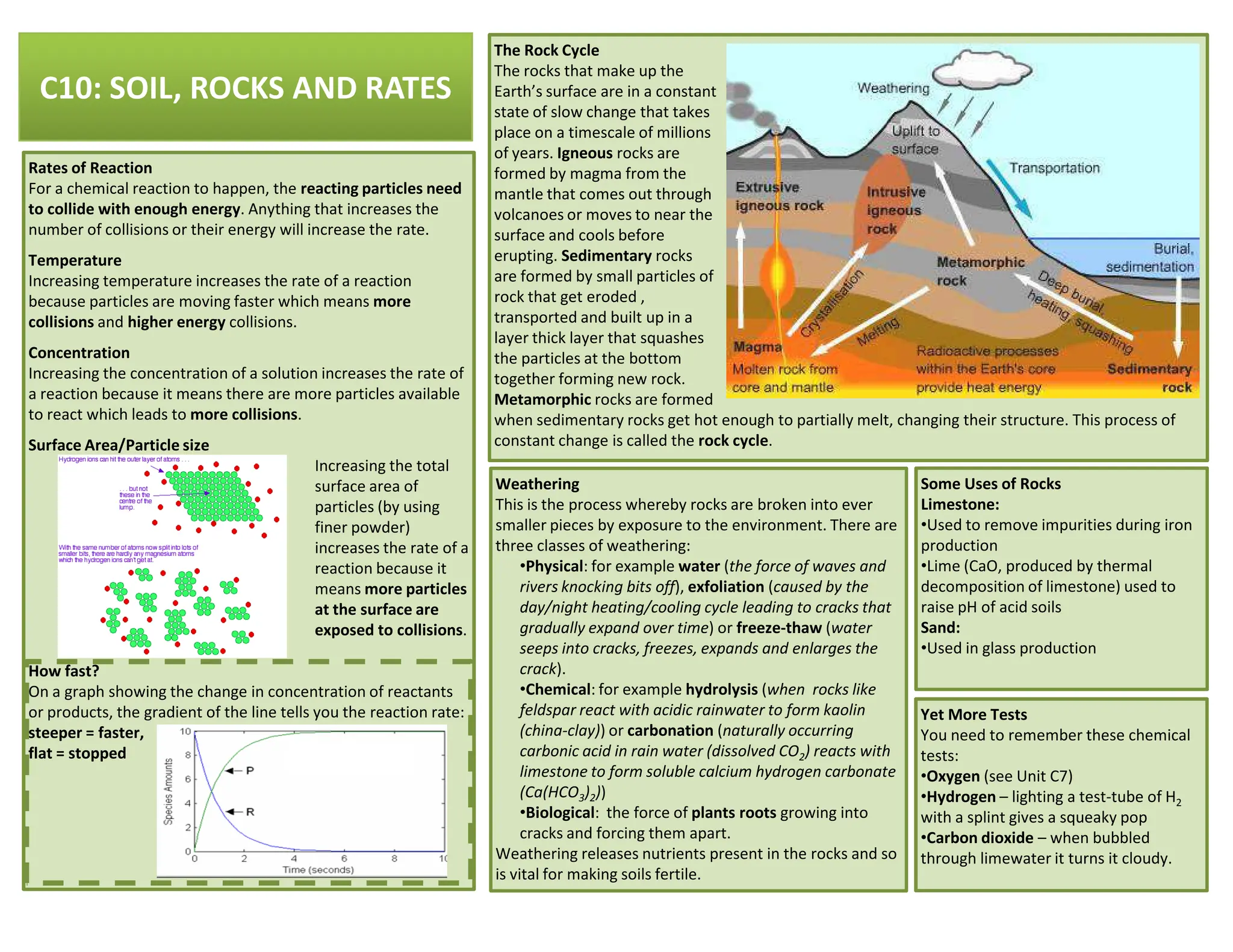
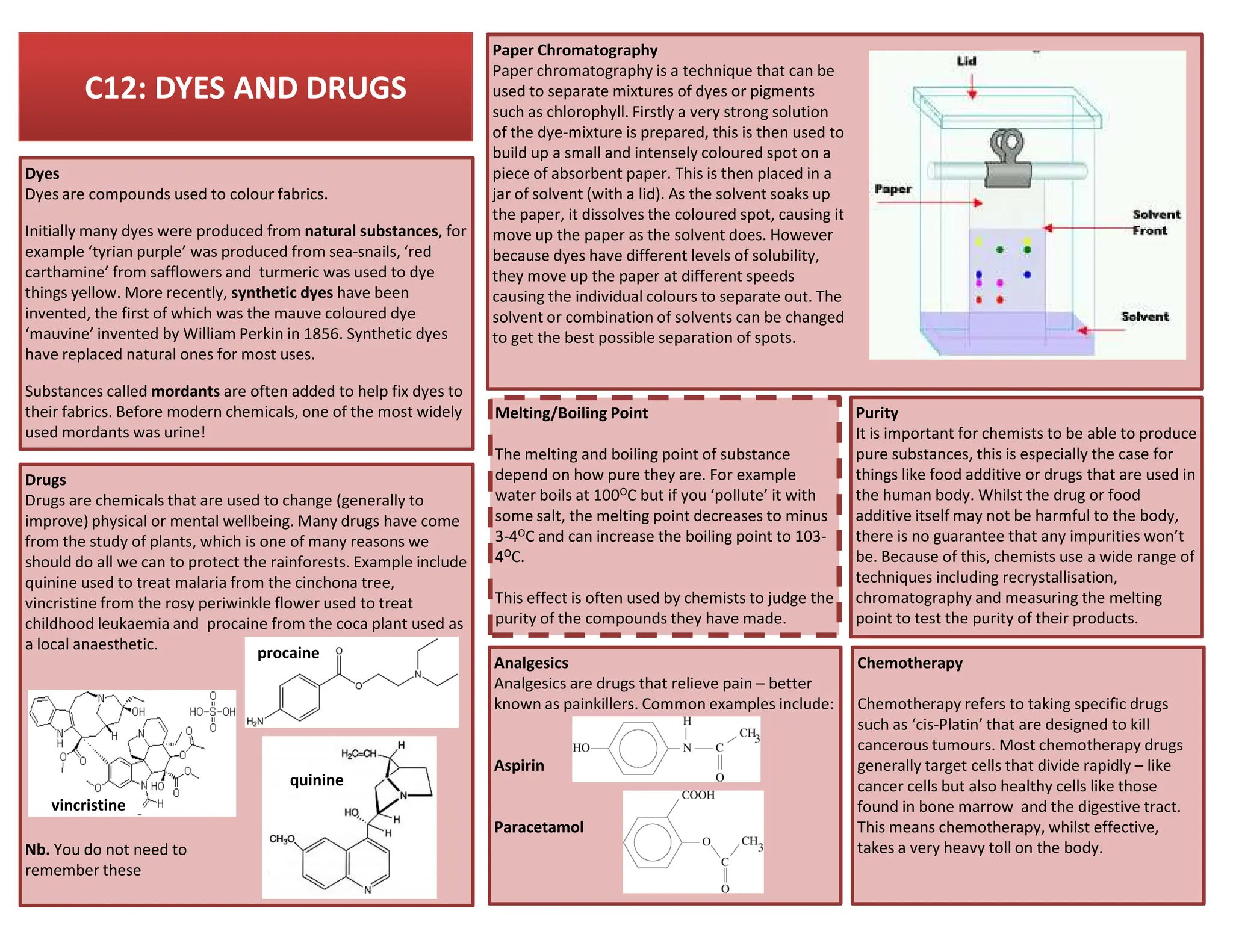
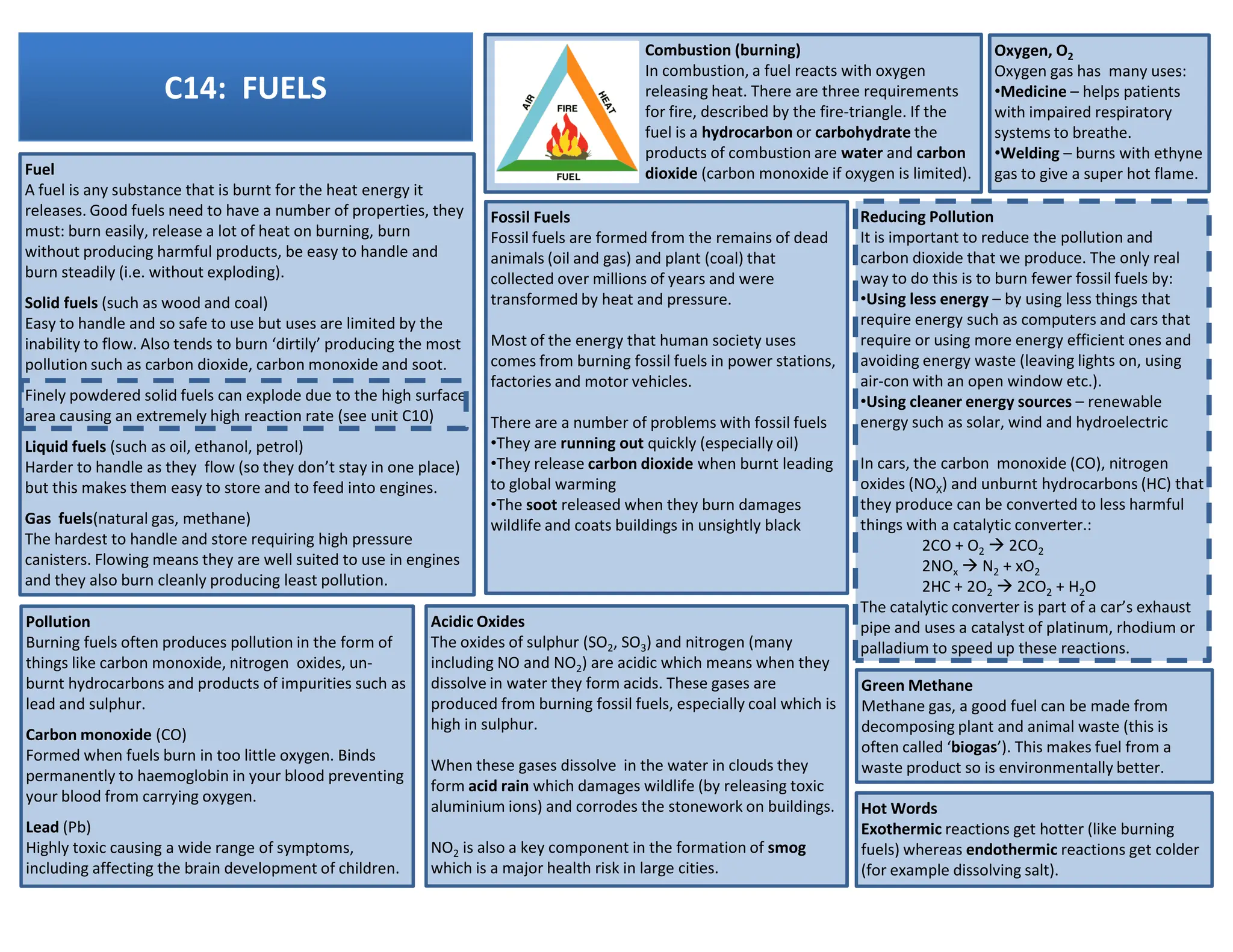
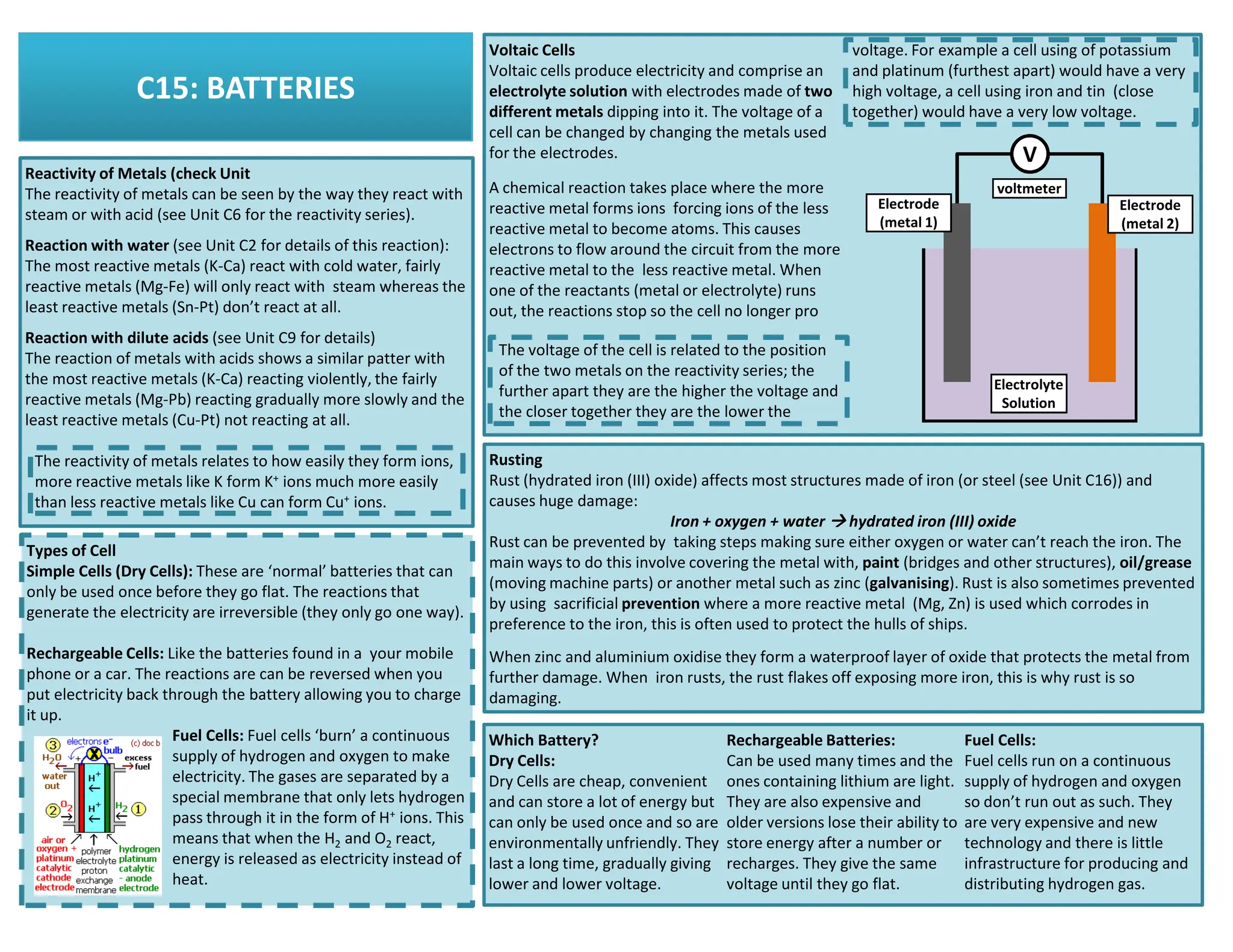
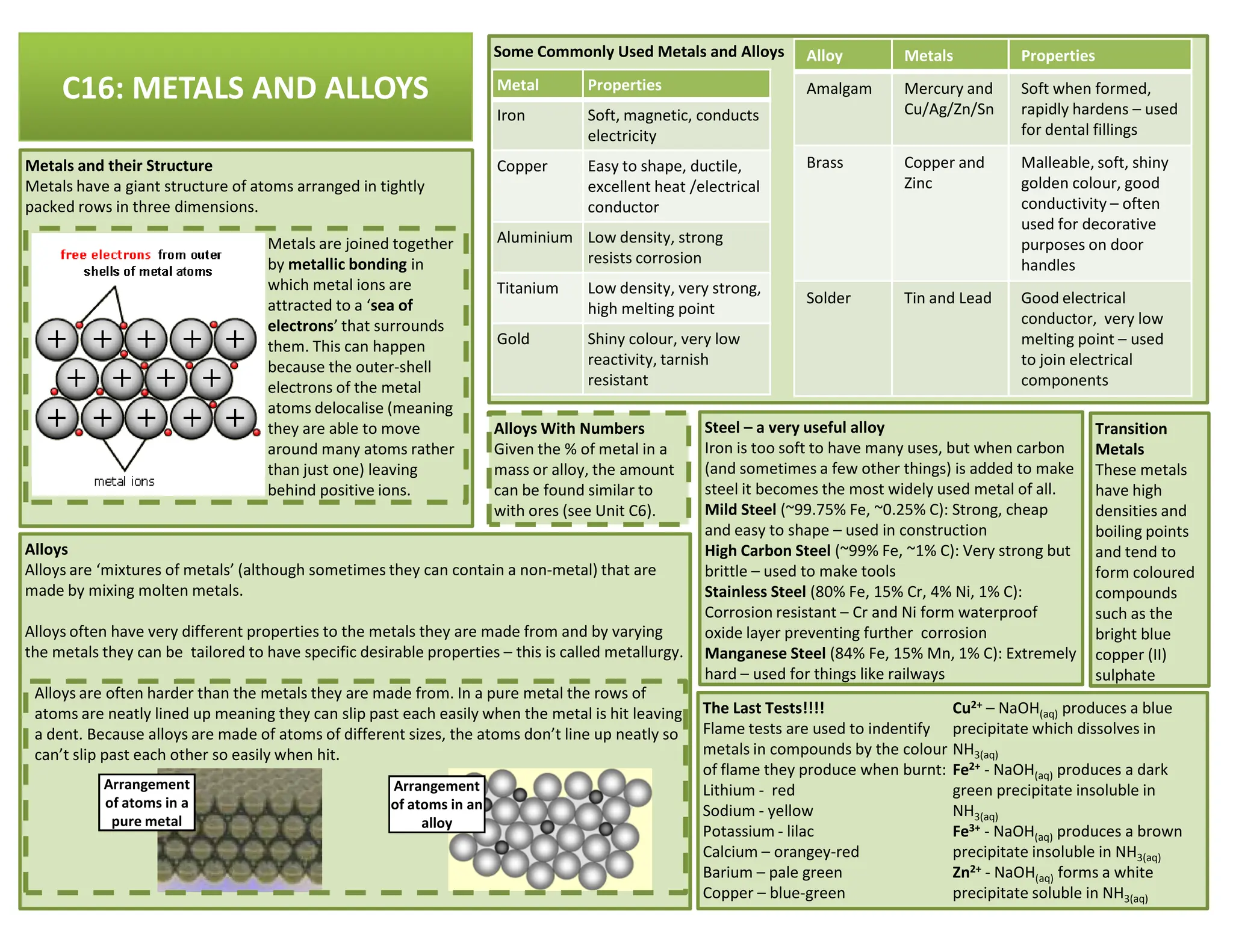
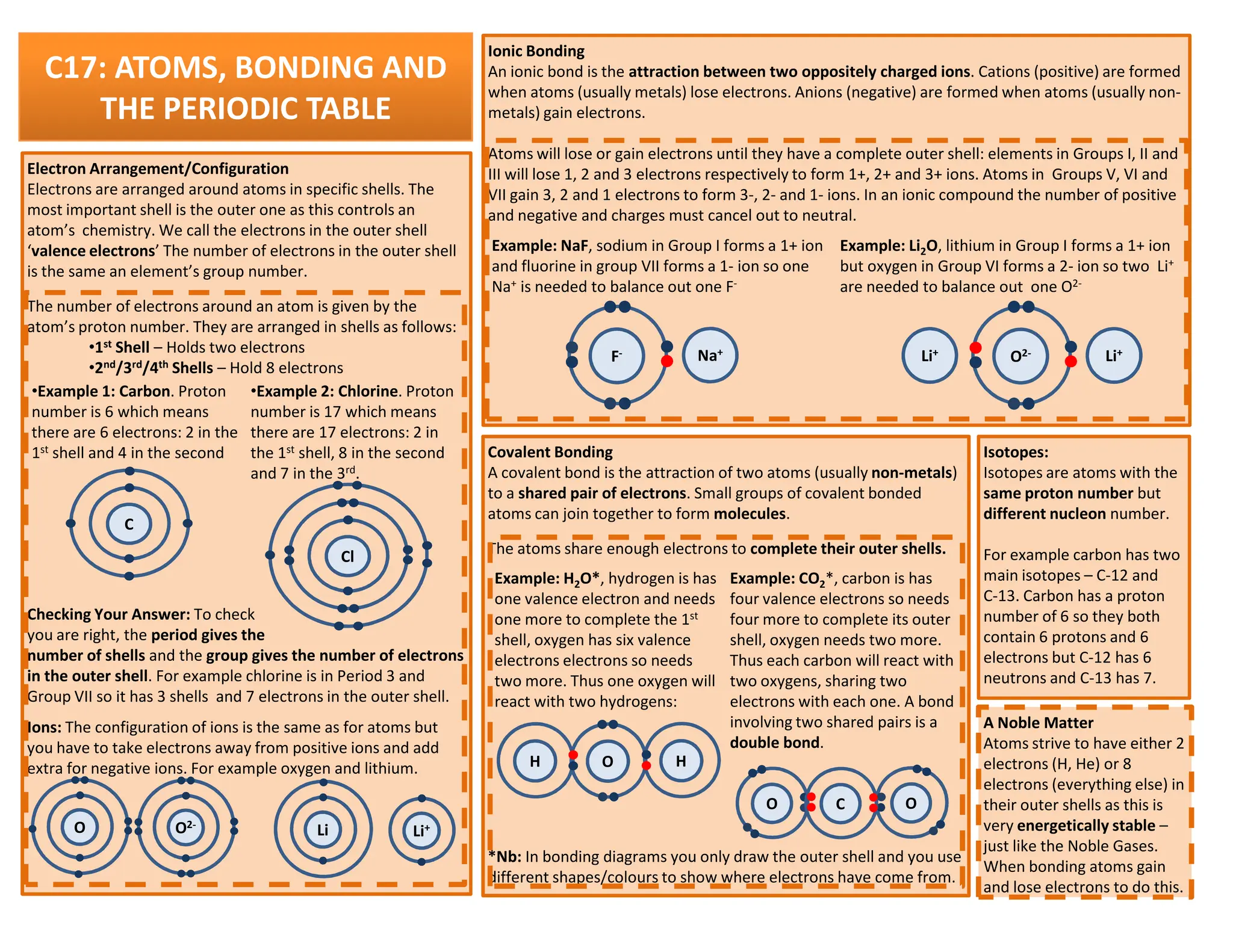
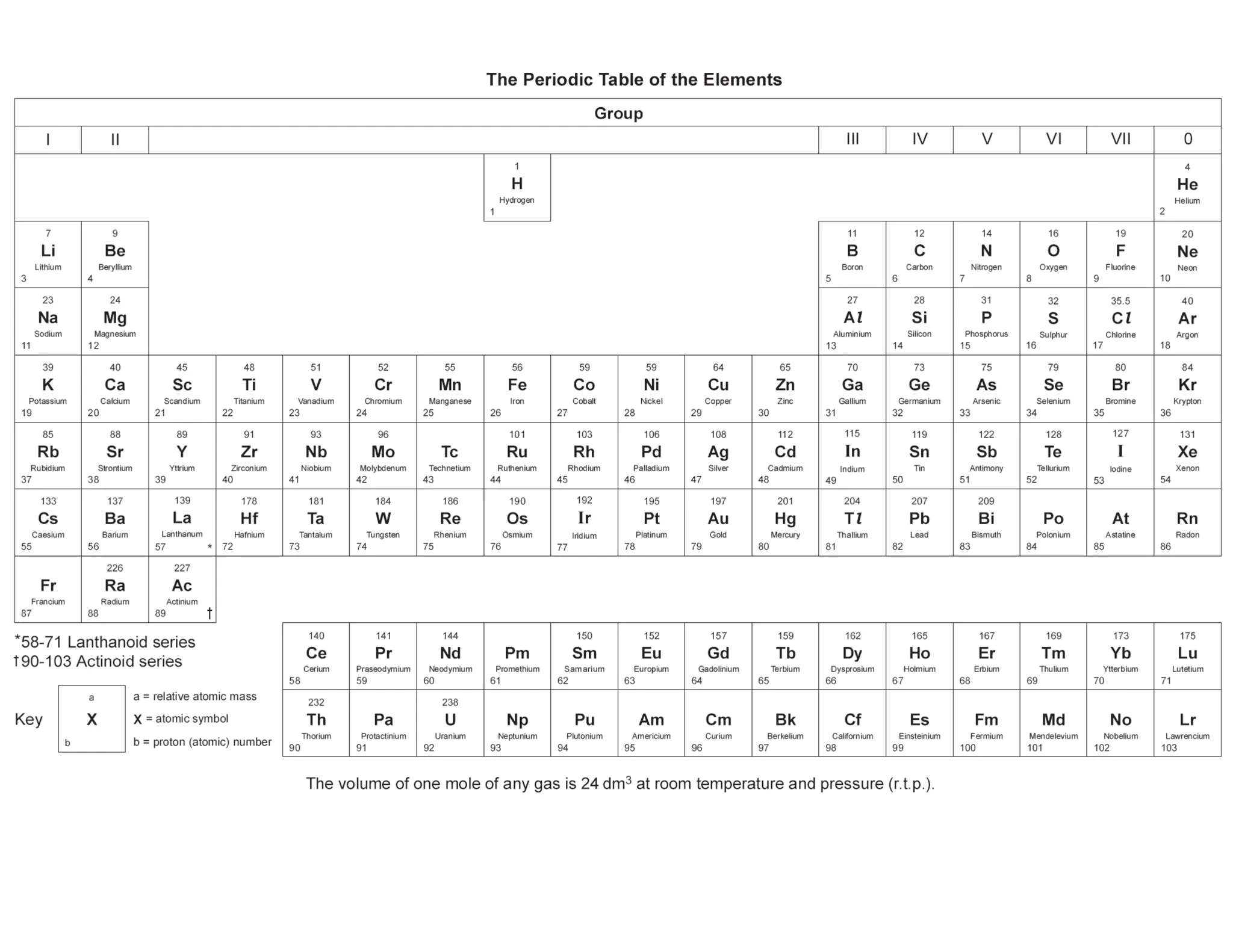
This revision guide is intended for students studying the chemistry portion of the IGCSE coordinated science course, providing comprehensive material aligned with the syllabus while emphasizing the necessity for additional resources and active study methods. It covers key concepts such as atomic structure, chemical bonding, periodic table classifications, hydrocarbon chemistry, and plant-derived carbohydrates and proteins. The guide encourages the use of diverse techniques for effective revision, including making notes, practicing past exam questions, and using online resources.