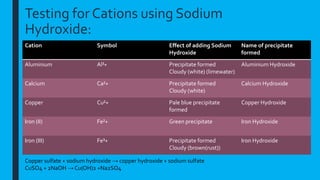
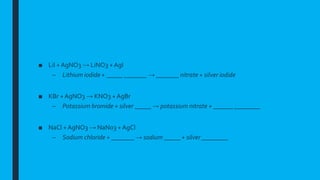
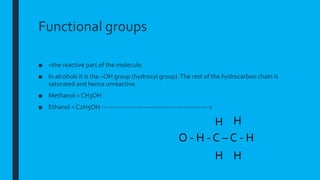
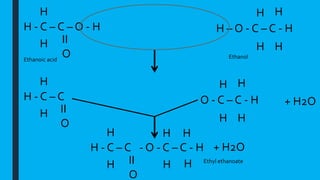
This document provides information on qualitative analysis in chemistry. It discusses qualitative versus quantitative analysis and describes tests to identify various ions including aluminum, calcium, copper, iron(II), iron(III), ammonium, chloride, bromide, and iodide. It also discusses using sodium hydroxide solution to test for cations, qualitative tests before quantitative tests, flame tests, and testing water for hardness. The key points covered in 3 sentences are: qualitative analysis identifies what substances are present while quantitative determines amounts, common cation tests use sodium hydroxide and anion tests use acids and silver nitrate, and hard water contains magnesium and calcium ions which affect lathering of soap.