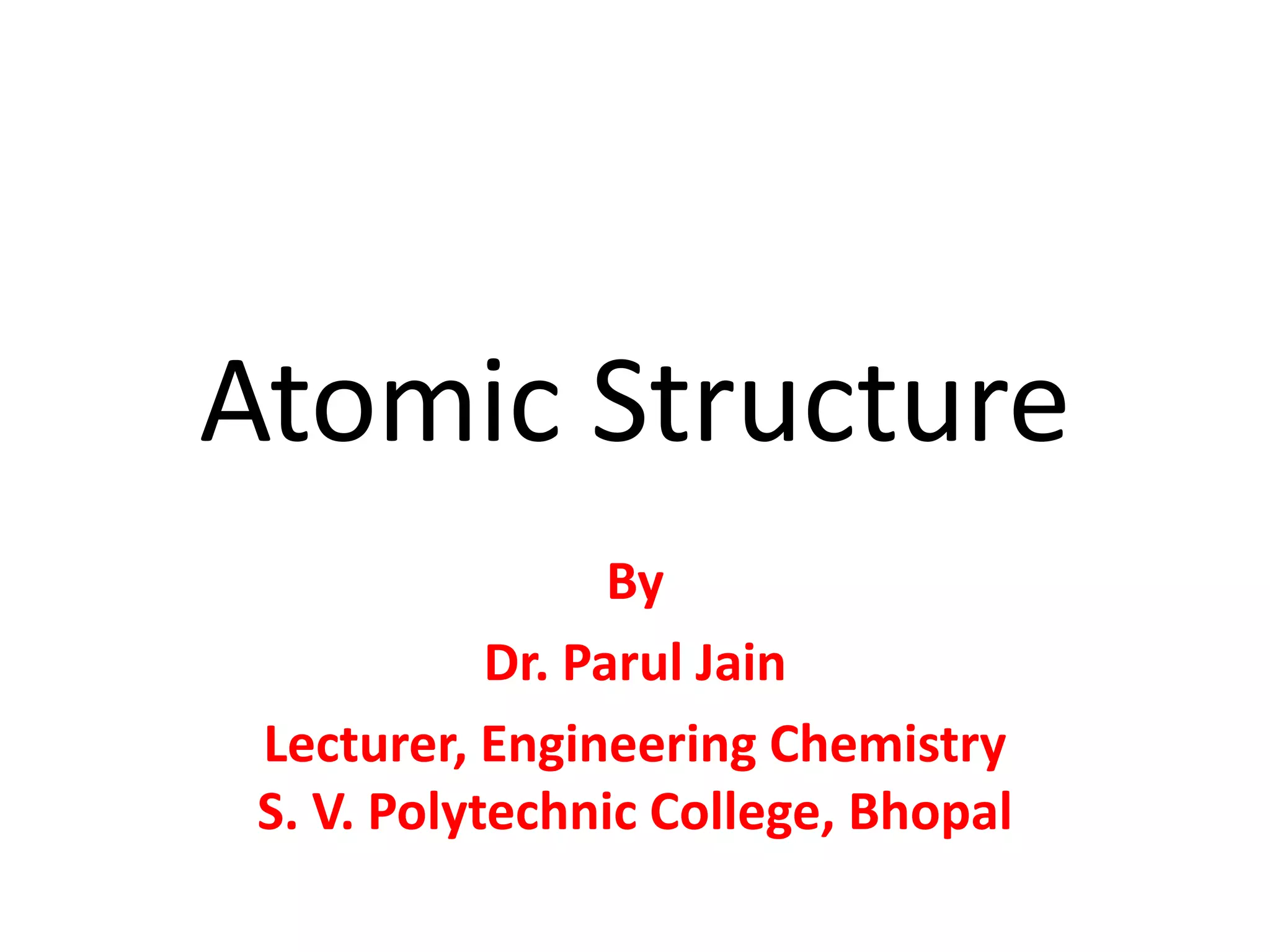
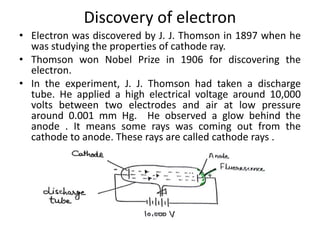
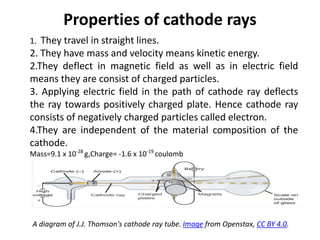
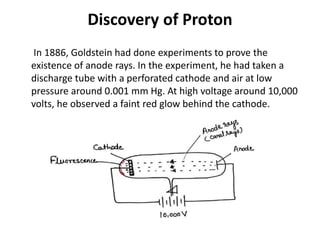
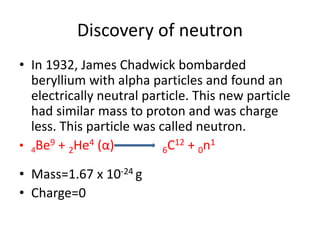
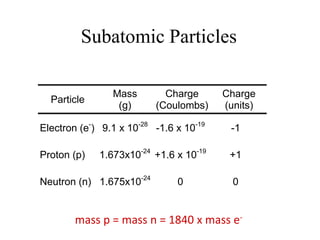
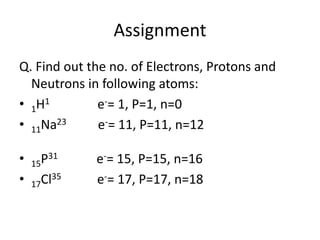
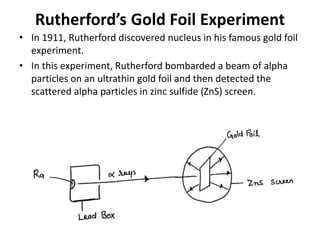
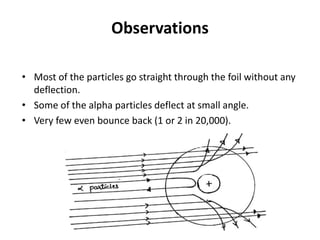
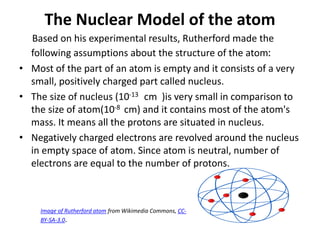
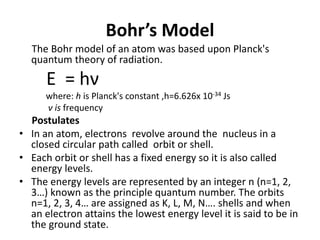
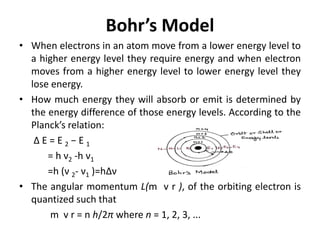
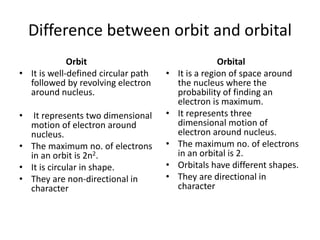
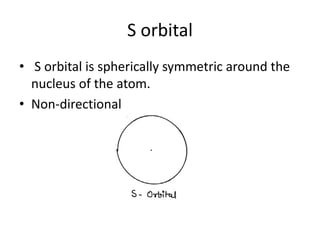
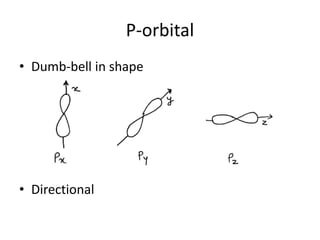
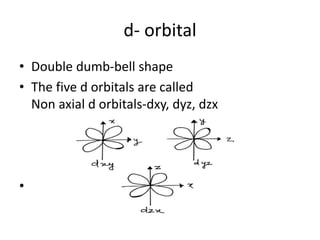
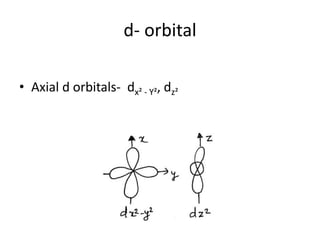
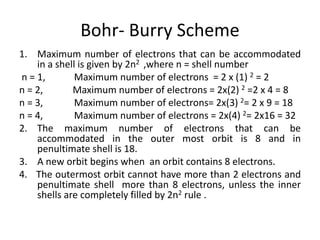
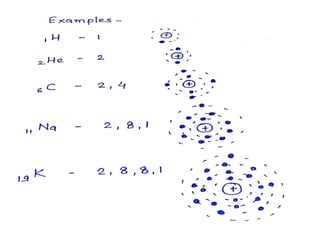
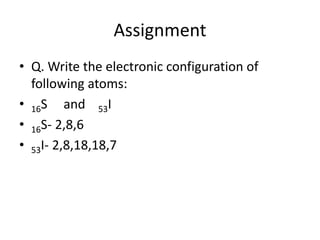
This document discusses atomic structure and the discovery of subatomic particles. It describes J.J. Thomson's discovery of the electron in cathode rays in 1897. The discovery of the proton in anode rays by Goldstein in 1886 is also discussed. The discovery of the neutron by Chadwick in 1932 when bombarding beryllium with alpha particles is summarized. Rutherford's gold foil experiment in 1911 is briefly described, which provided evidence for the nuclear model of the atom with electrons orbiting a small, dense nucleus. Bohr's model improved upon Rutherford's by introducing electron energy levels and orbits. Key concepts like electron configuration, atomic number, mass number, and orbital shapes are defined.