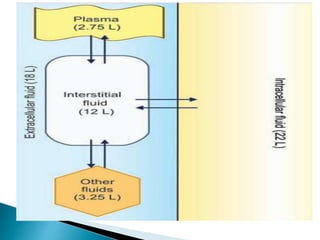
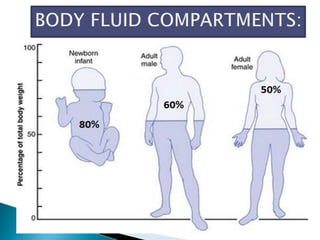
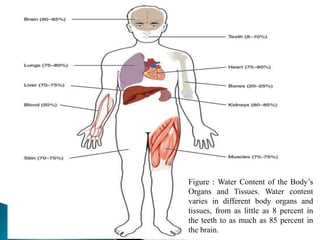
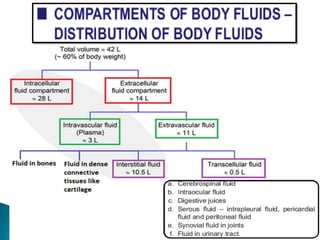
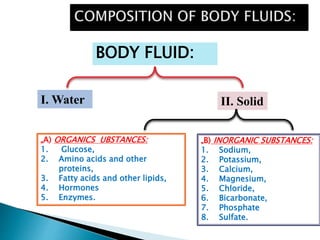
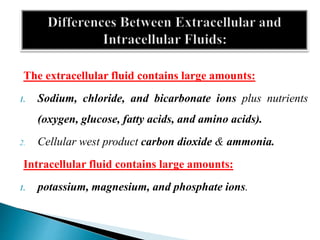
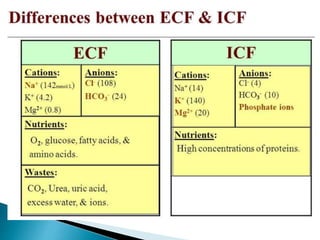
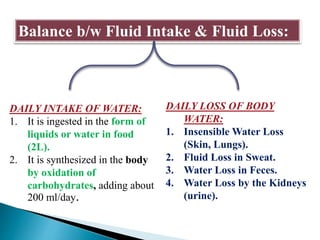
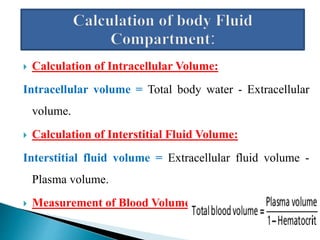
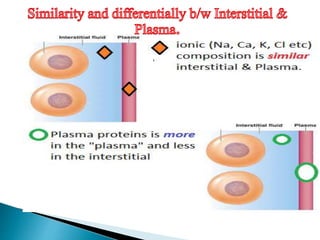
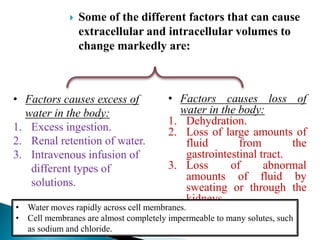
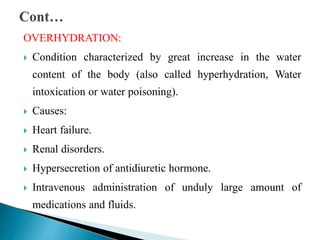
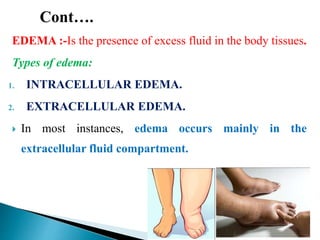
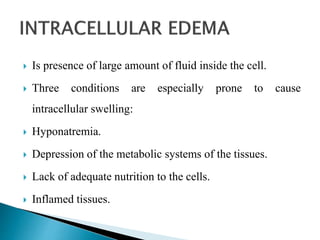
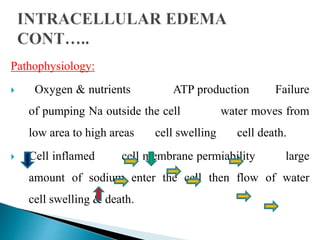
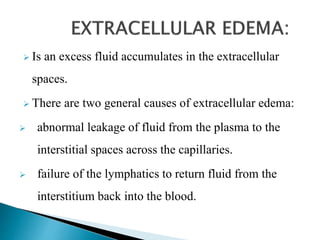
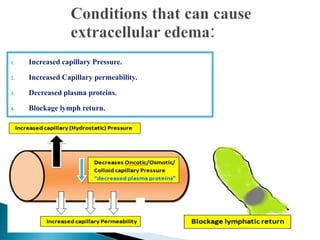
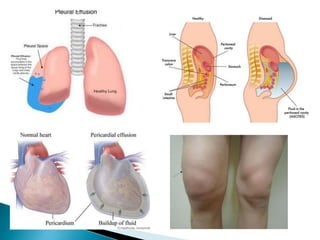
The document discusses the composition and regulation of body fluids in humans. About 60% of the adult human body is made up of fluid, with two thirds located intracellularly and one third extracellularly. Homeostasis and transport mechanisms precisely regulate fluid levels. Fluid content depends on factors like age, gender and obesity level. Dehydration is more common in children due to immature regulatory systems. Imbalances in fluid levels can cause conditions like edema or dehydration.