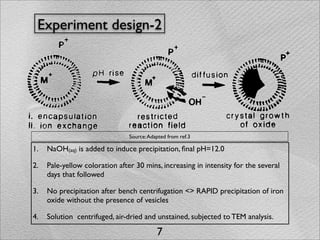
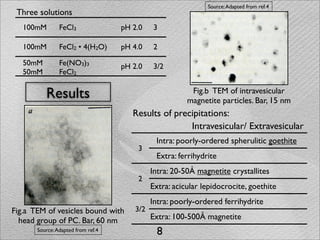
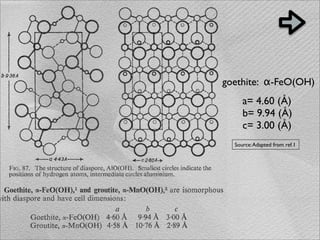
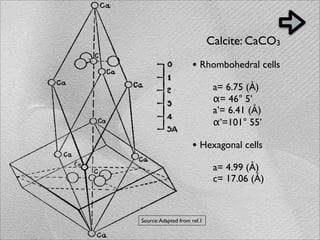
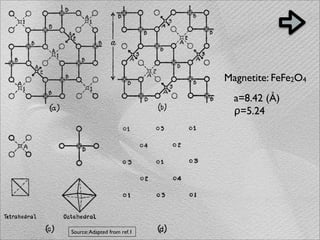
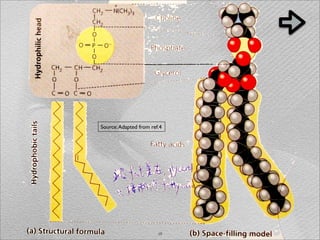
The document summarizes a 1986 Nature article about an experiment using phospholipid vesicles as a model system to study biomineralization. The experiment investigated the biomineralization of iron oxide within vesicles. Iron solutions were encapsulated within vesicles and precipitation was induced by raising the pH. Transmission electron microscopy revealed the formation of magnetite crystallites within the vesicles, demonstrating that vesicles can control mineralization through an intracellular environment.