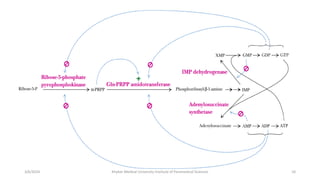
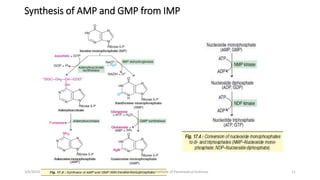
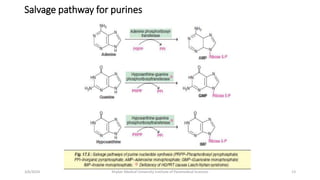
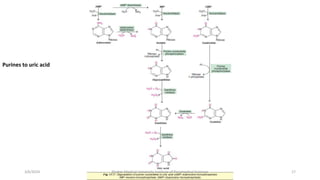
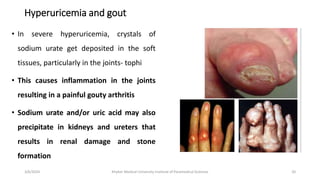
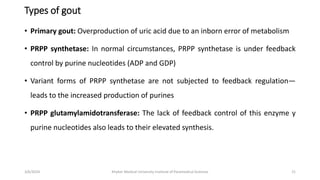
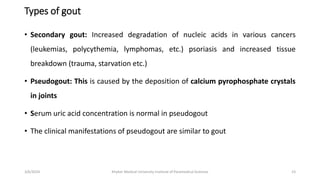
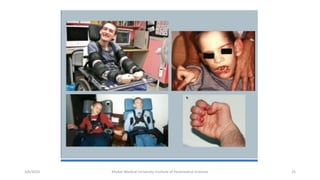
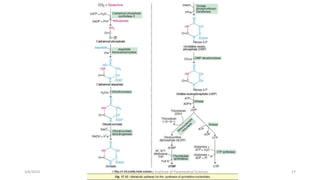
The document discusses nucleotide metabolism. It describes that nucleotides consist of a nitrogenous base, pentose sugar, and phosphate. The pentose is D-ribose in RNA and 2-deoxy D-ribose in DNA. Nucleotides are components of nucleic acids and involved in metabolic reactions. It provides details on the de novo biosynthesis of purines and pyrimidines, which both start with ribose-5-phosphate and involve multiple enzymatic steps to form the nucleotide precursors IMP and UMP. Salvage pathways are also discussed which allow recycling of purine and pyrimidine bases.

![Regulation of de novo synthesis
• The significance of regulation:
1. Meet the need of the body, without wasting.
2. AMP and GMP control their respective synthesis from IMP by a feedback
mechanism, [GTP]=[ATP]
8
3/6/2024 Khyber Medical University Institute of Paramedical Sciences](https://image.slidesharecdn.com/5-240306073000-1567a589/85/Nucleotide-metabolism-biochemistry-lecture-8-320.jpg)