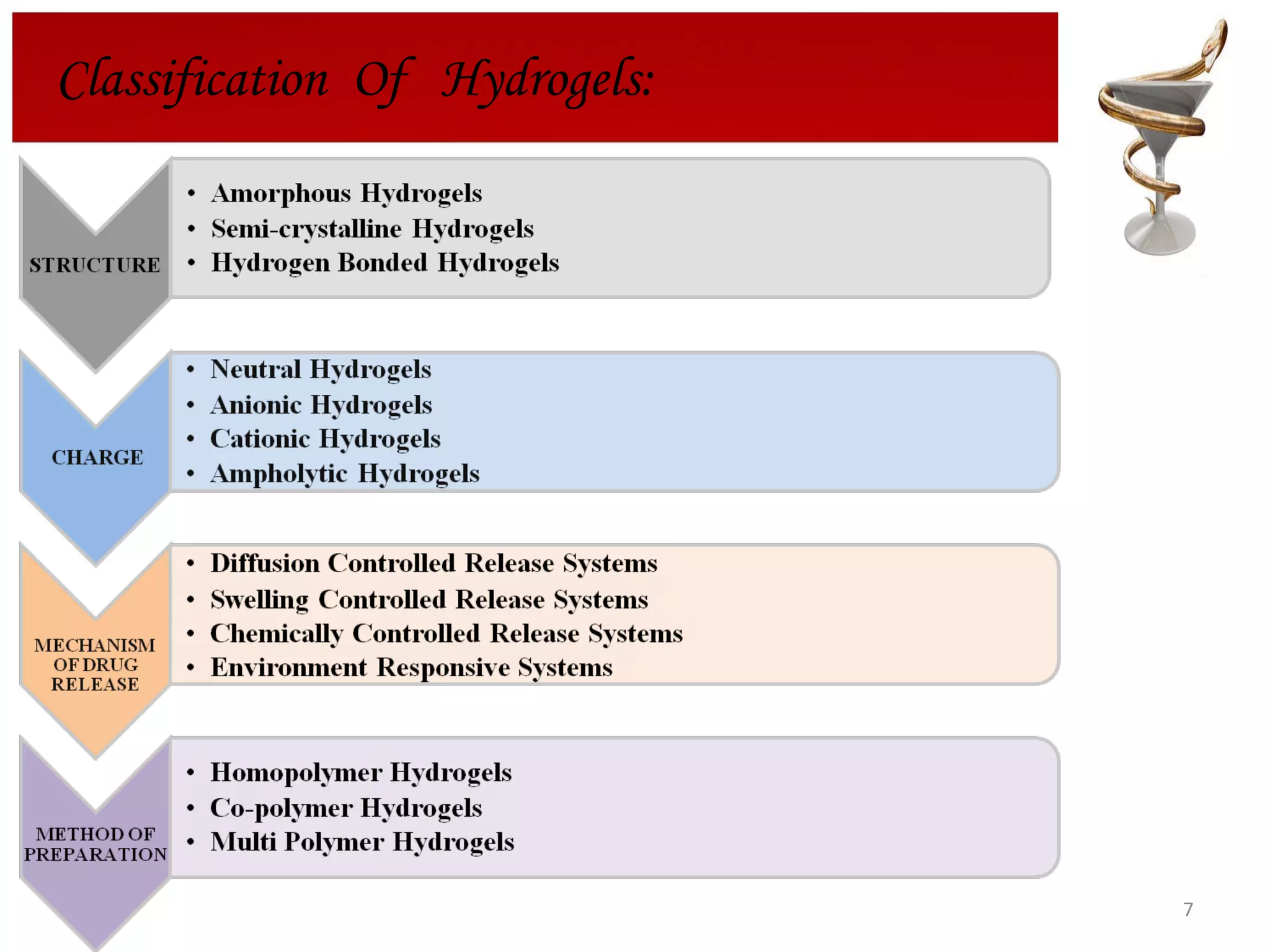
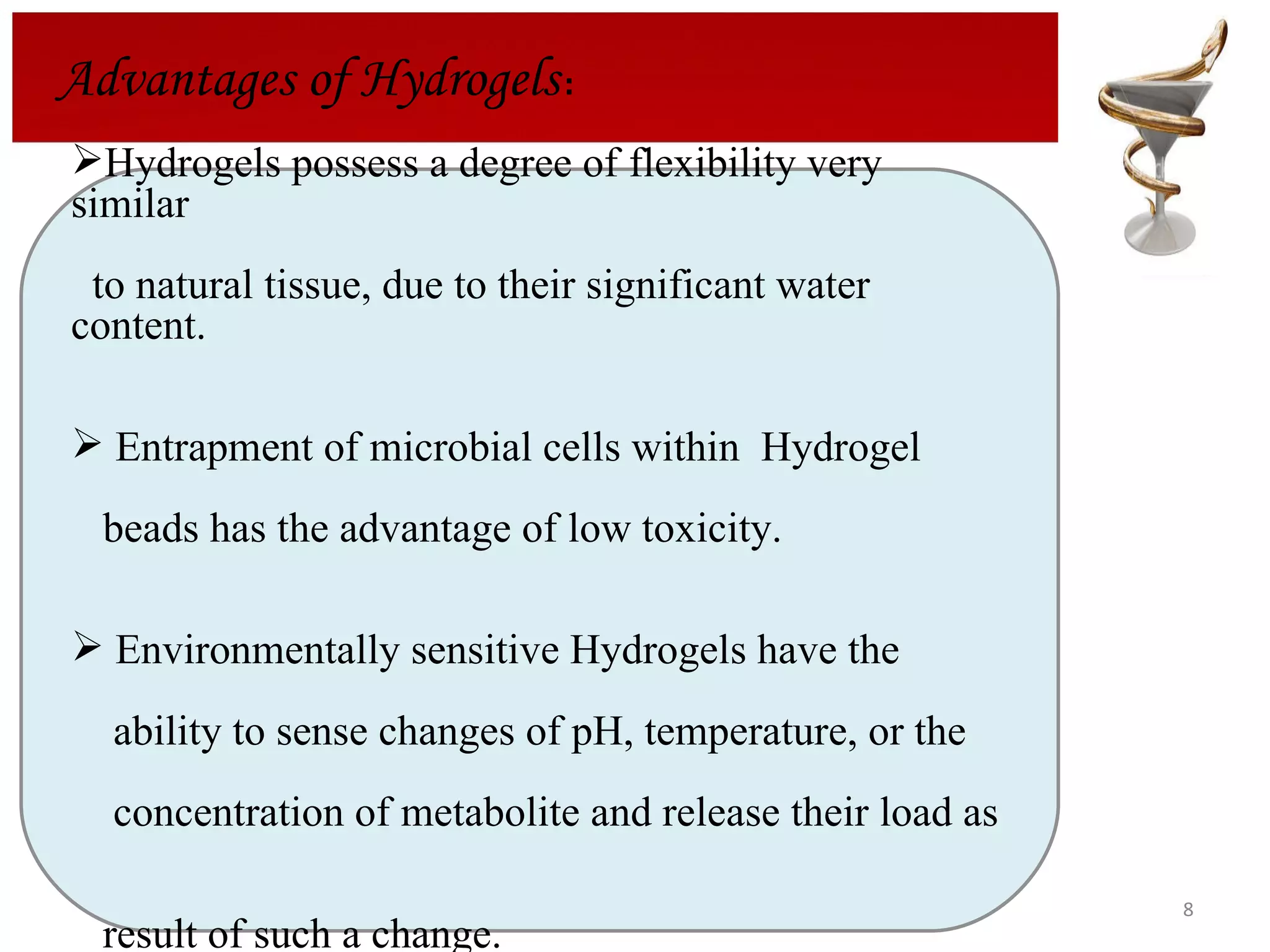
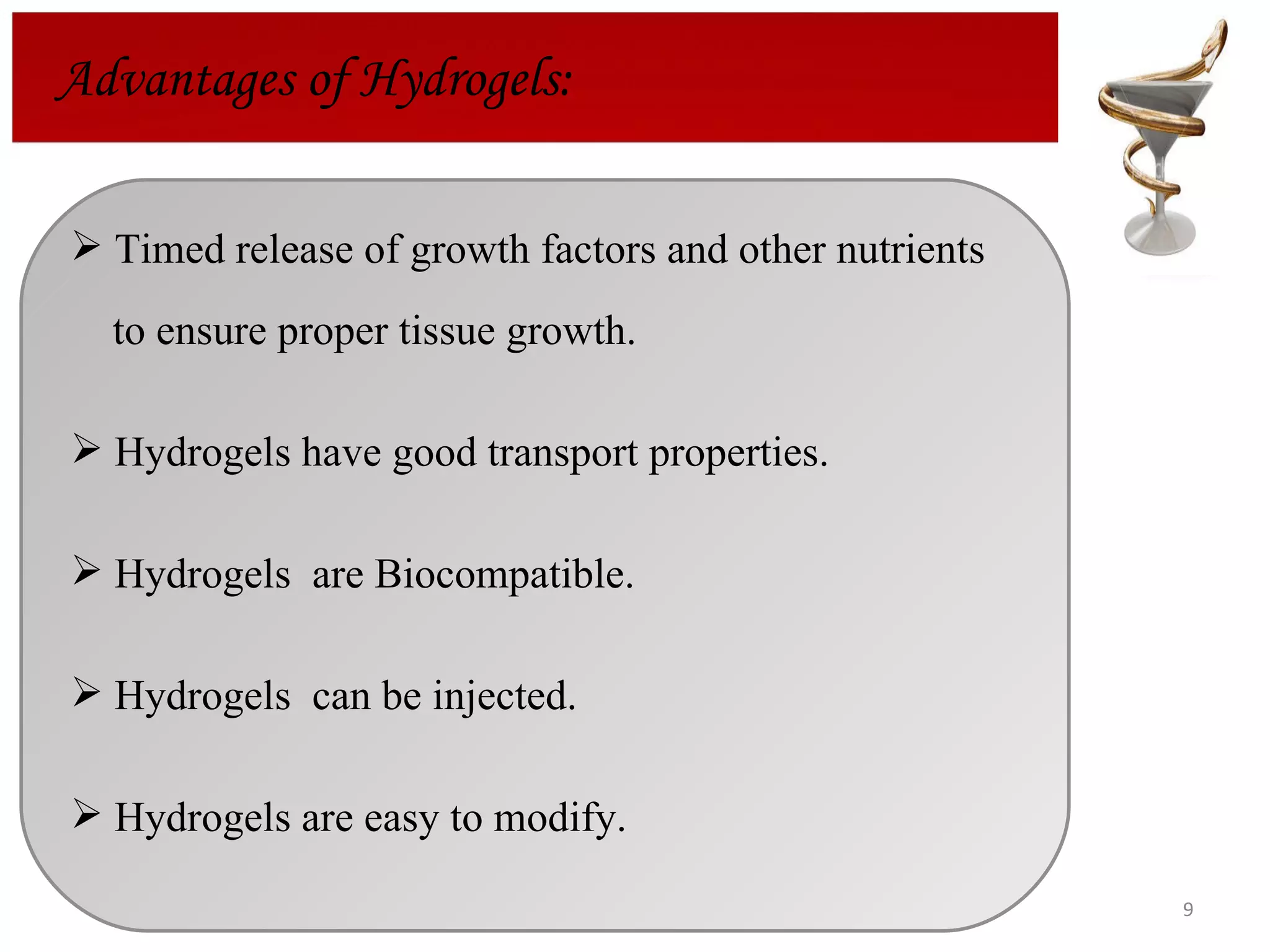
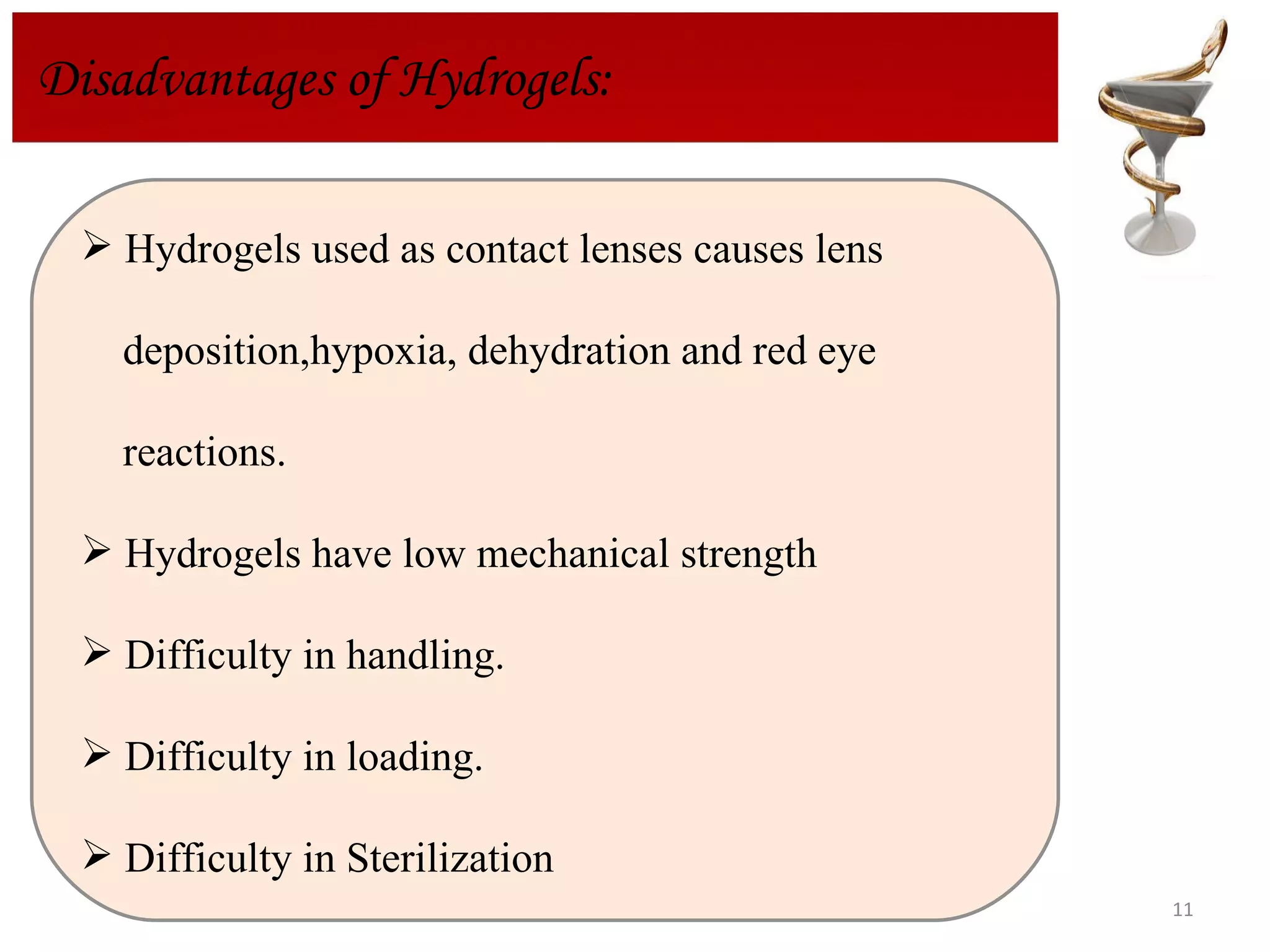
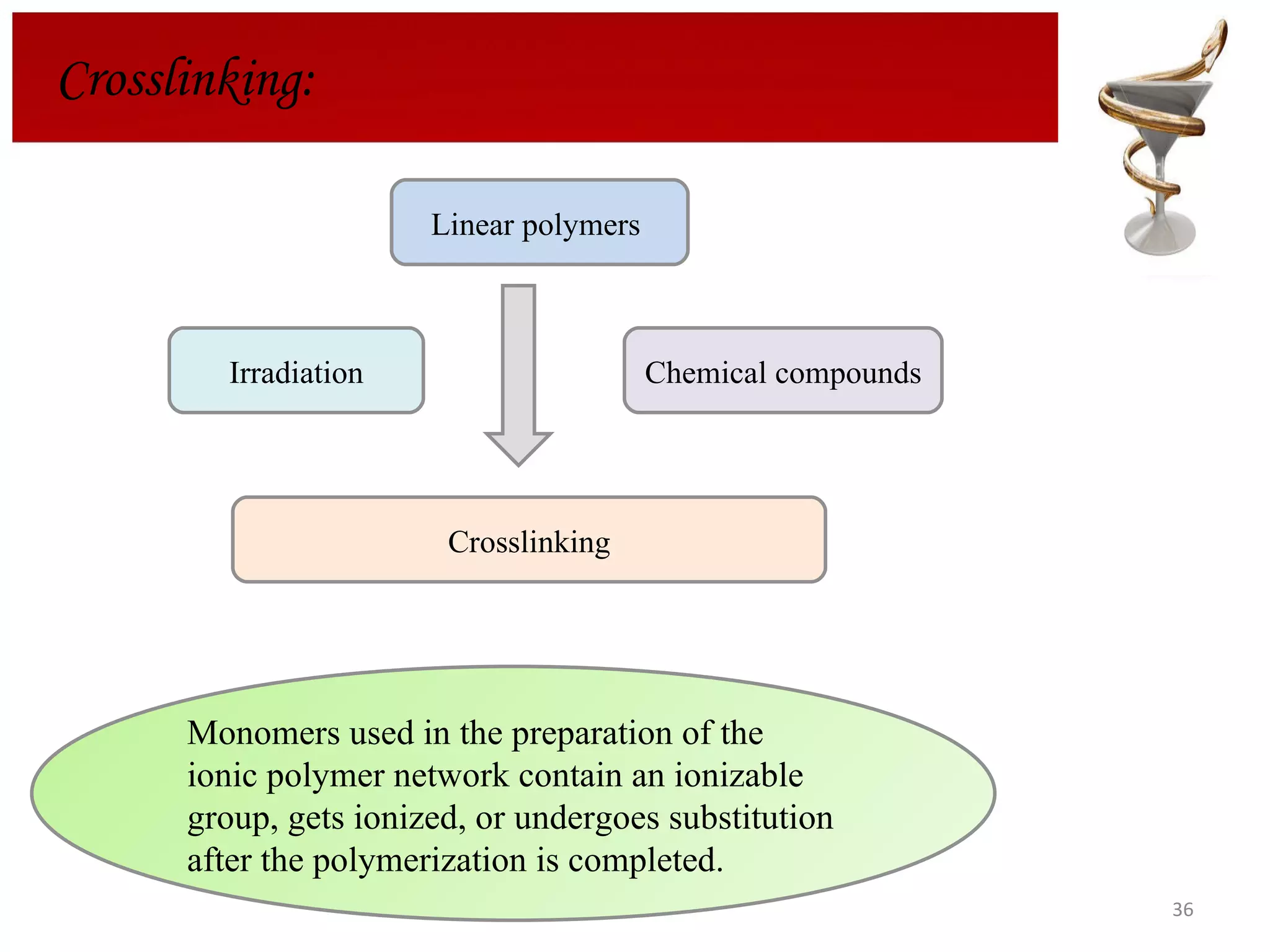
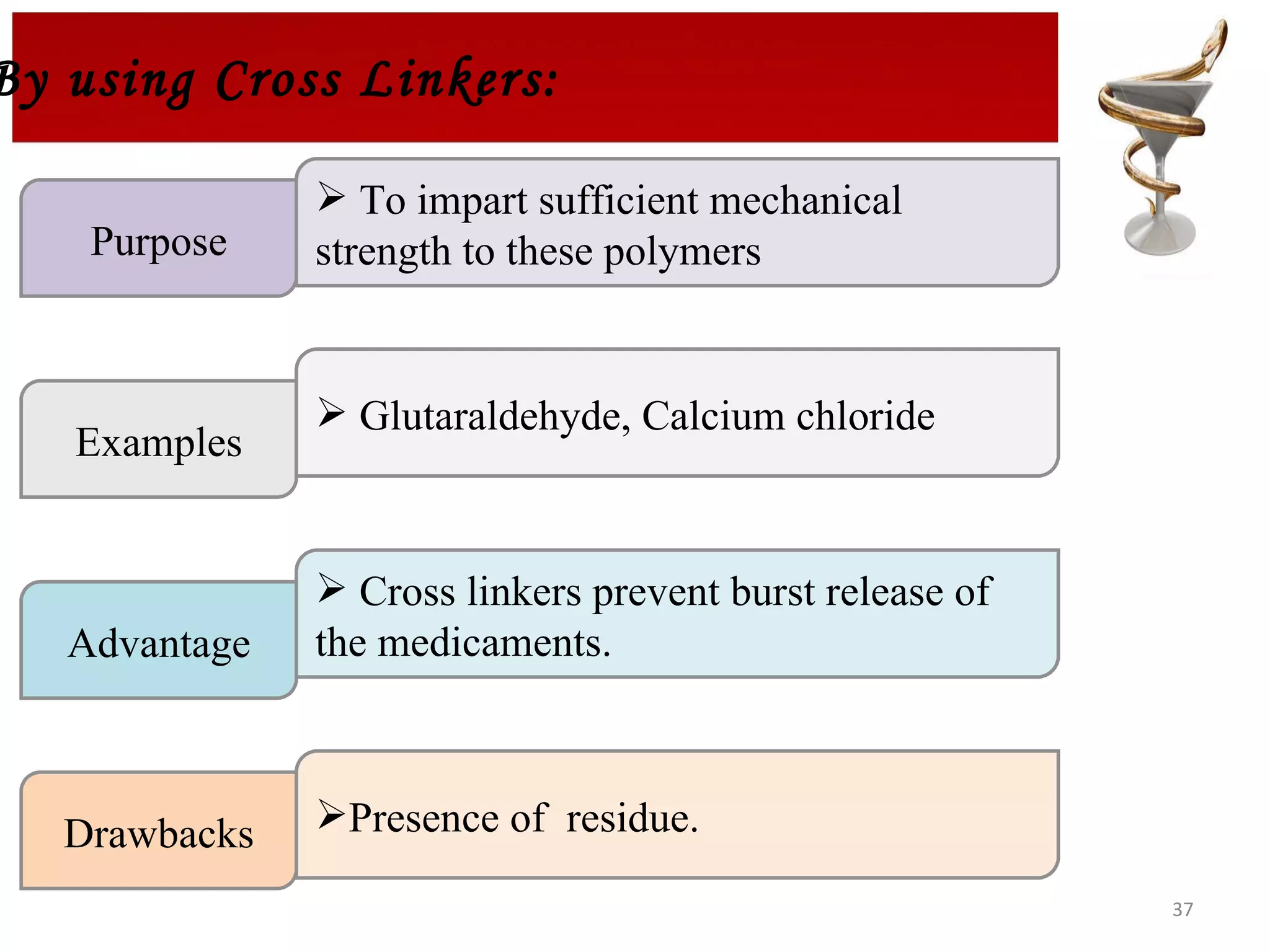
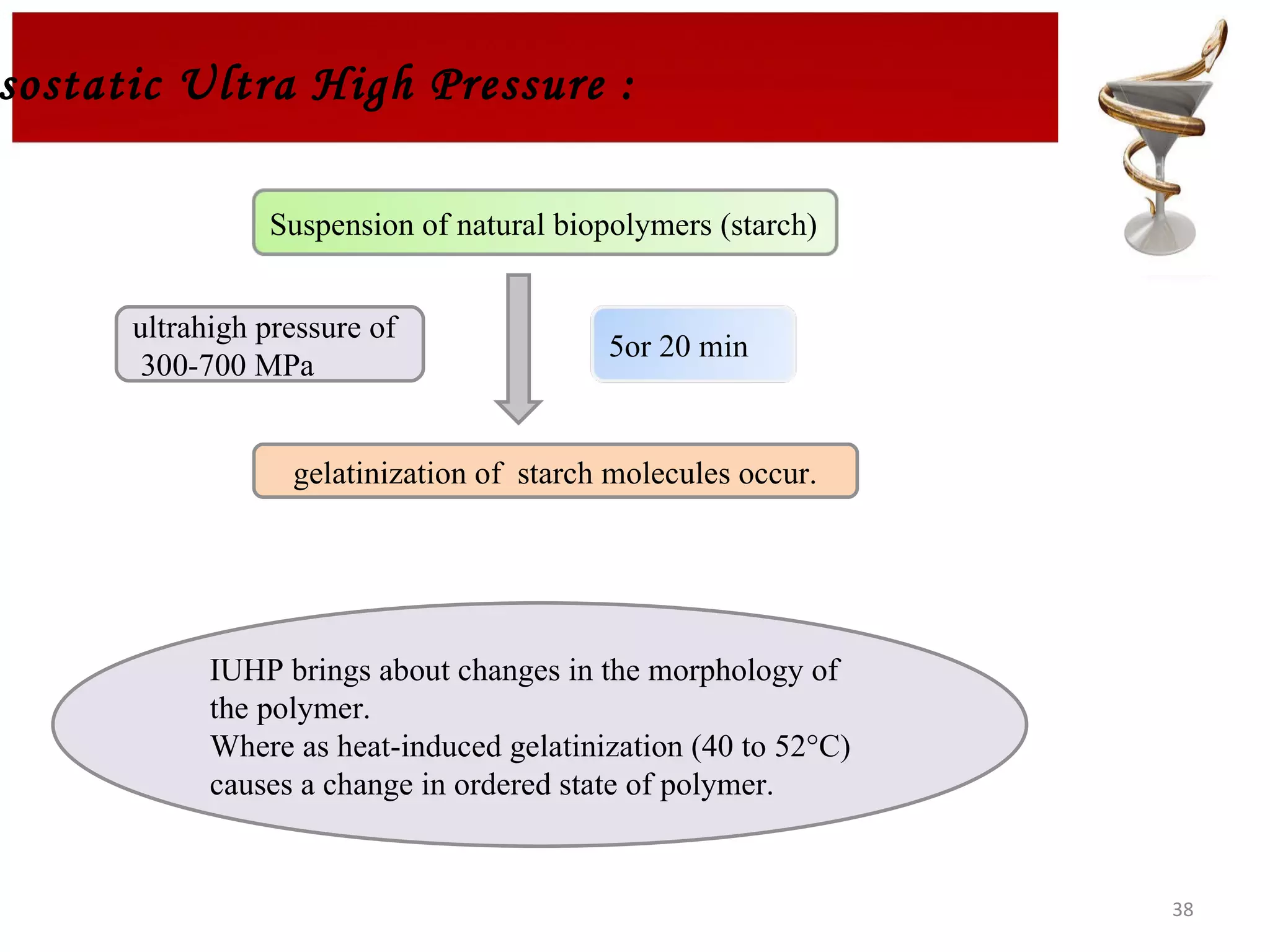
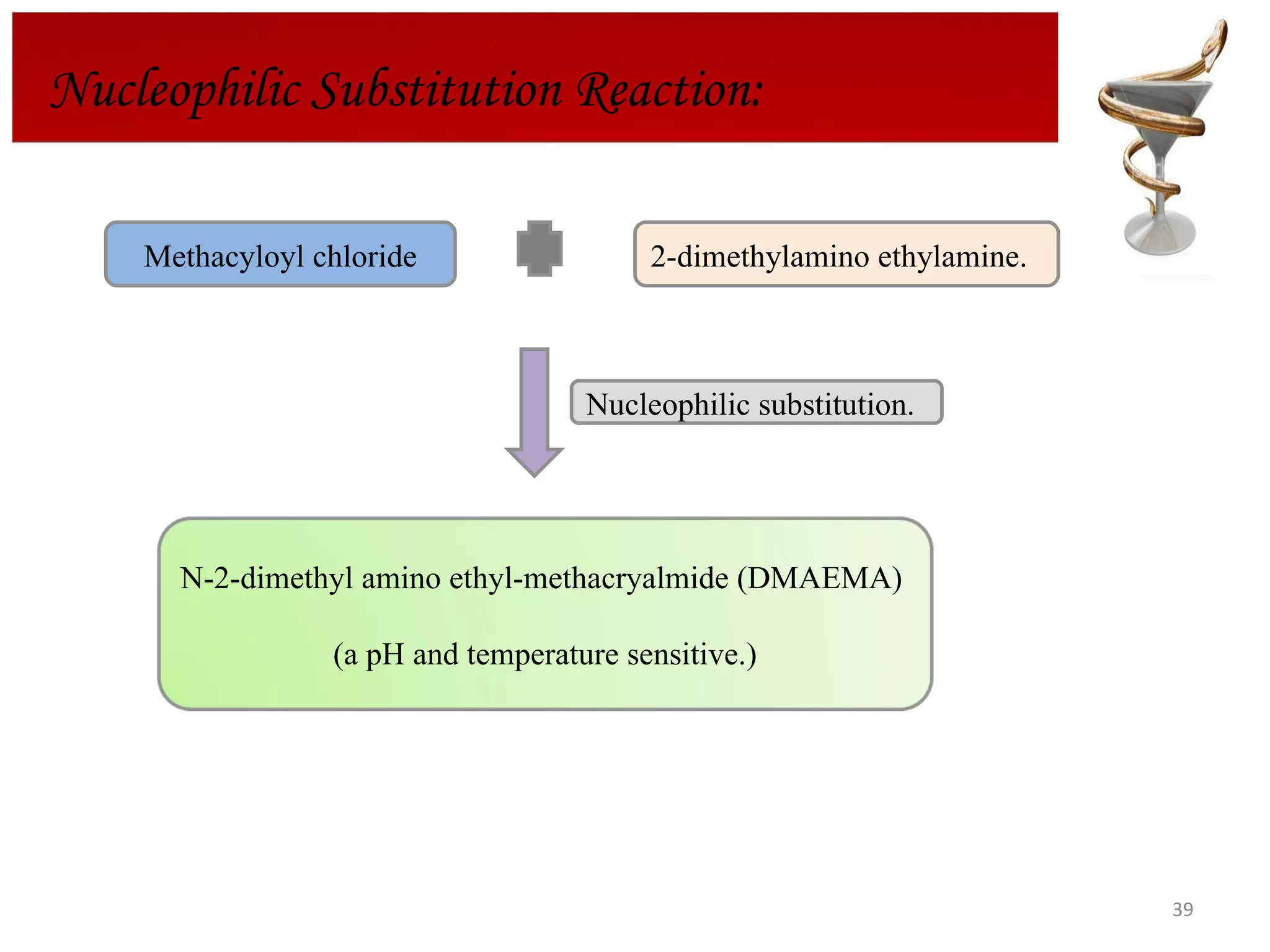
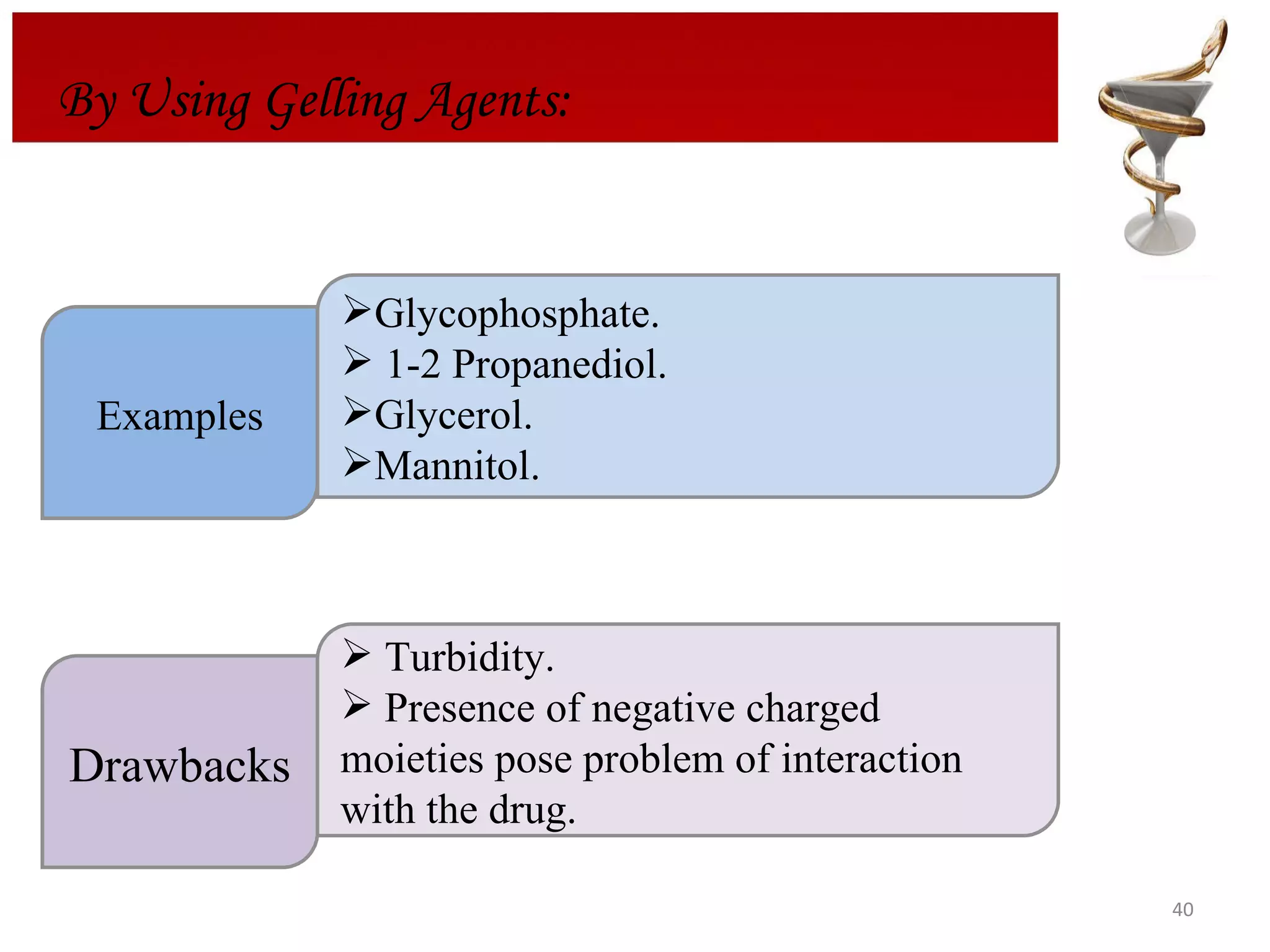
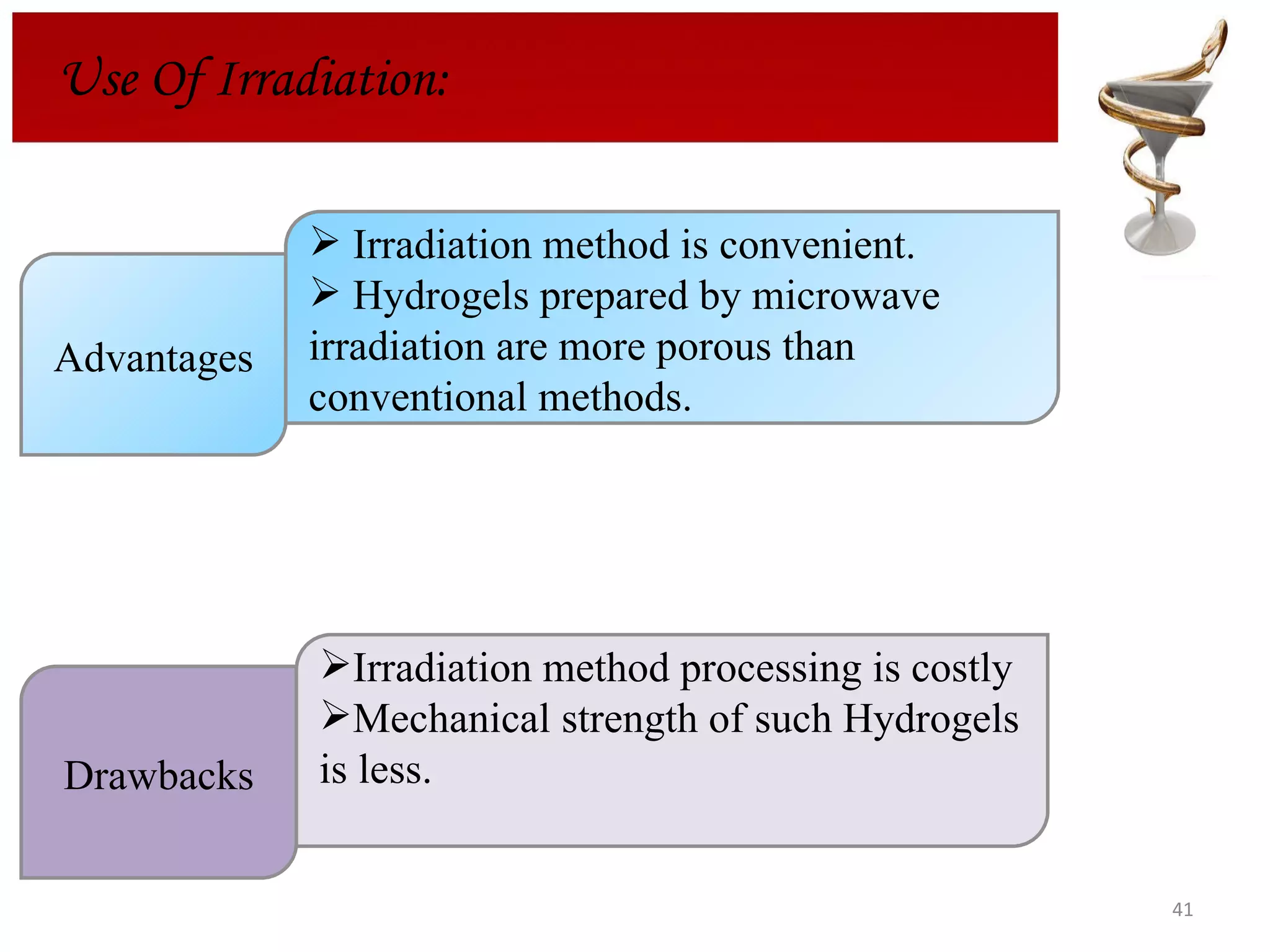
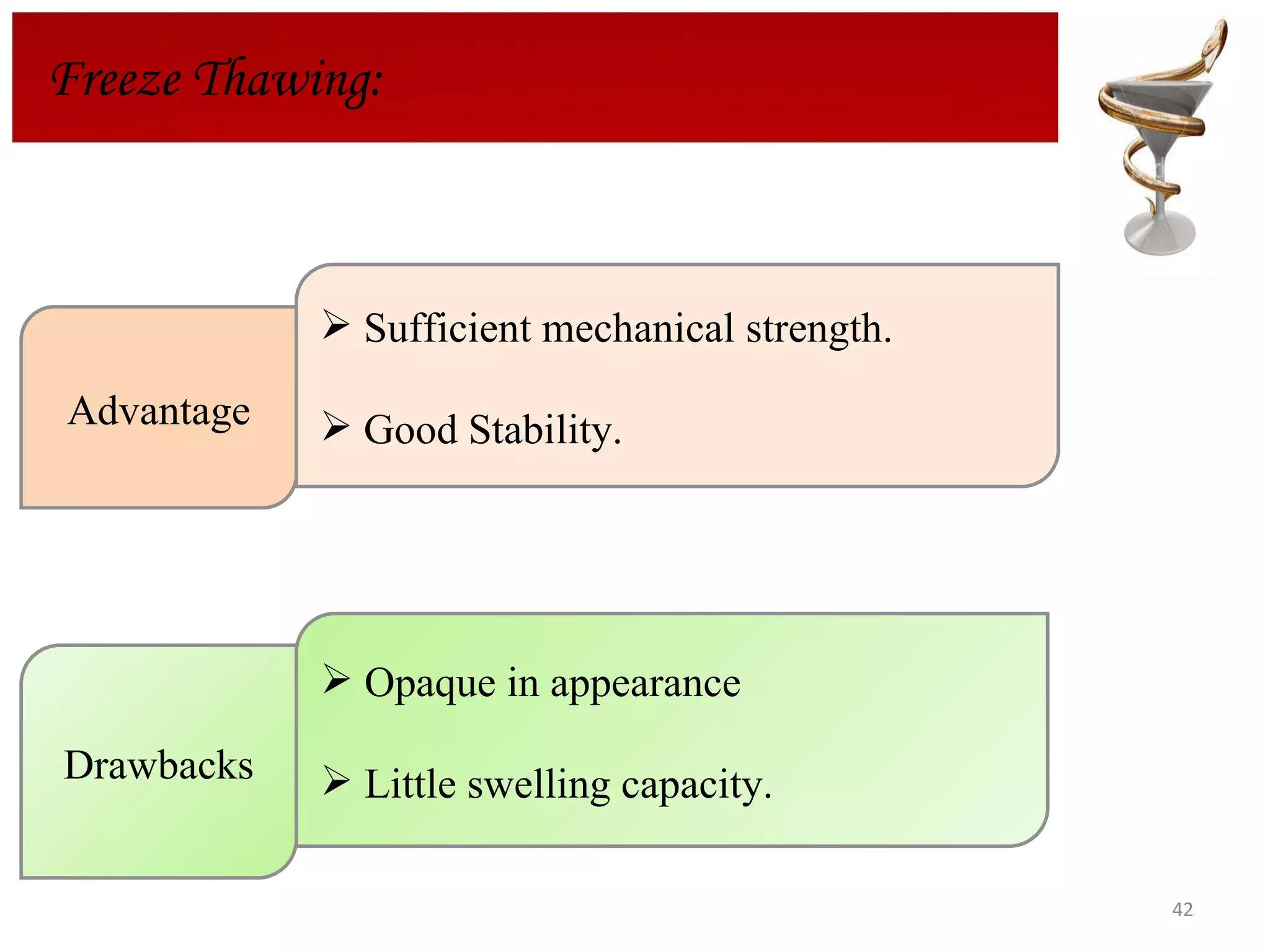
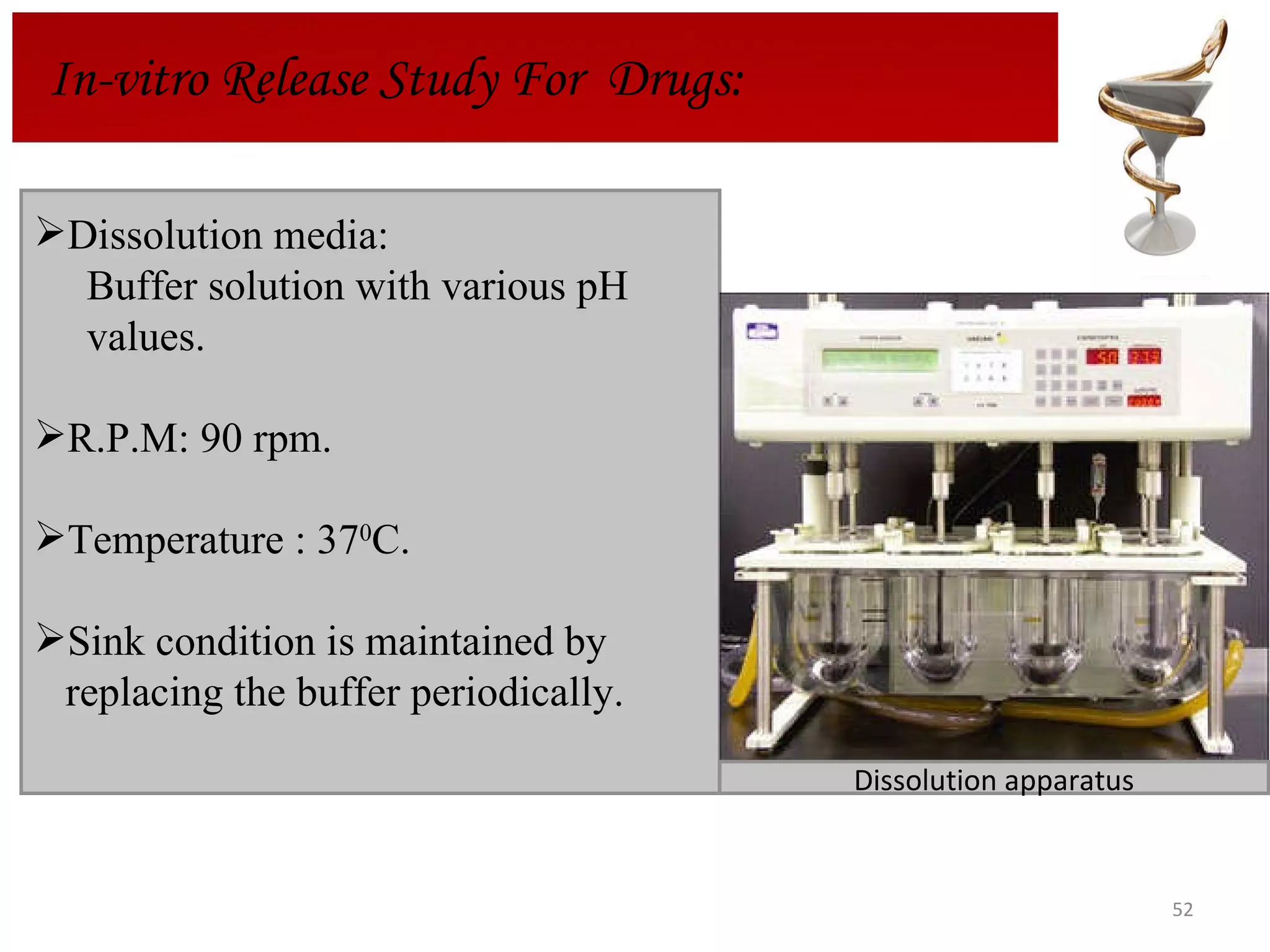
The document discusses hydrogels, including their classification, advantages, disadvantages, types, monomers used in synthesis, methods of preparation, characterization, uses, and pharmaceutical applications. Hydrogels are crosslinked polymer networks that can absorb large amounts of water. They are biocompatible and can be used for controlled drug release in applications such as contact lenses, wound dressings, and tissue engineering scaffolds.