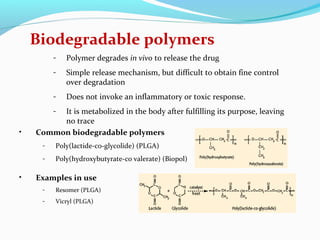
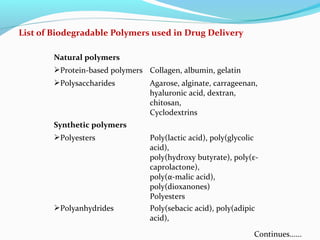
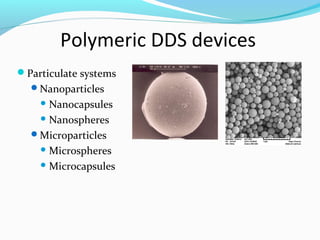
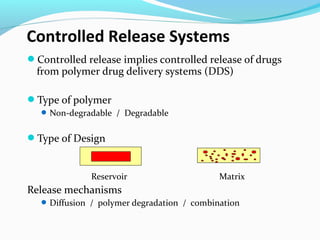
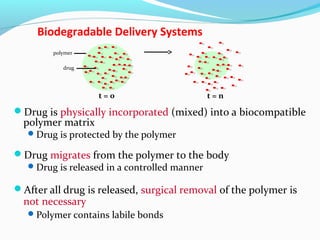
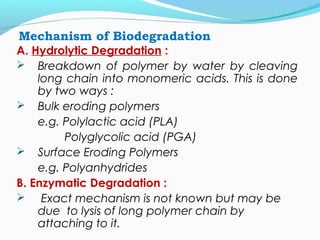
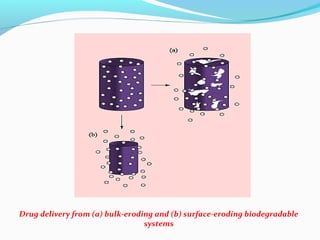
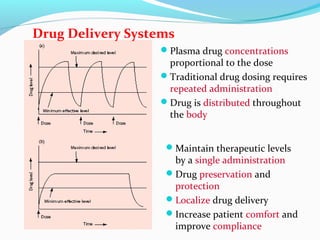
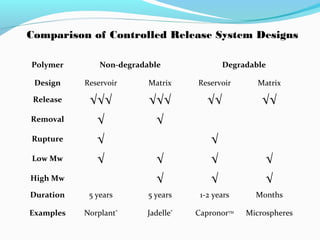
This document discusses biodegradable polymeric delivery systems for drug delivery. It describes how biodegradable polymers can be used as carriers for proteins and peptides. Some key biodegradable polymers mentioned include poly(lactide-co-glycolide) and poly(hydroxybutyrate-co-valerate). The document discusses the characteristics of ideal polymers for drug delivery and criteria for polymer selection. It also provides details on controlled release mechanisms and compares delivery system designs.

![Polymer?
• Large molecule composed of a number of sub-units
- Natural e.g. alginates,
- synthetic e.g. poly(HMPA)
- Function governed by number and arrangement of constitutional repeat
units e.g. –[A-]n, -[A-B-]n, -[A-A] n-[B-B] m , --A-A-B-A-B-B-A-
• How are they made?
- Processing of natural products – alginates from seaweeds, celluloses
from plants
- Synthesis from chemical feedstocks – poly(olefins), nylons, poly(esters)
• How can they help?
- Protection of therapeutic compound during passage through body, as
encapsulant or carrier.
- Mediator or activator of controlled release](https://image.slidesharecdn.com/biodegradablepolymers-141028091659-conversion-gate01/85/Biodegradable-Polymers-3-320.jpg)