1) Biodegradable polymers are polymers that break down into non-toxic molecules via biological processes such as hydrolysis or enzymatic degradation. They are used for applications such as drug delivery where degradation is beneficial.
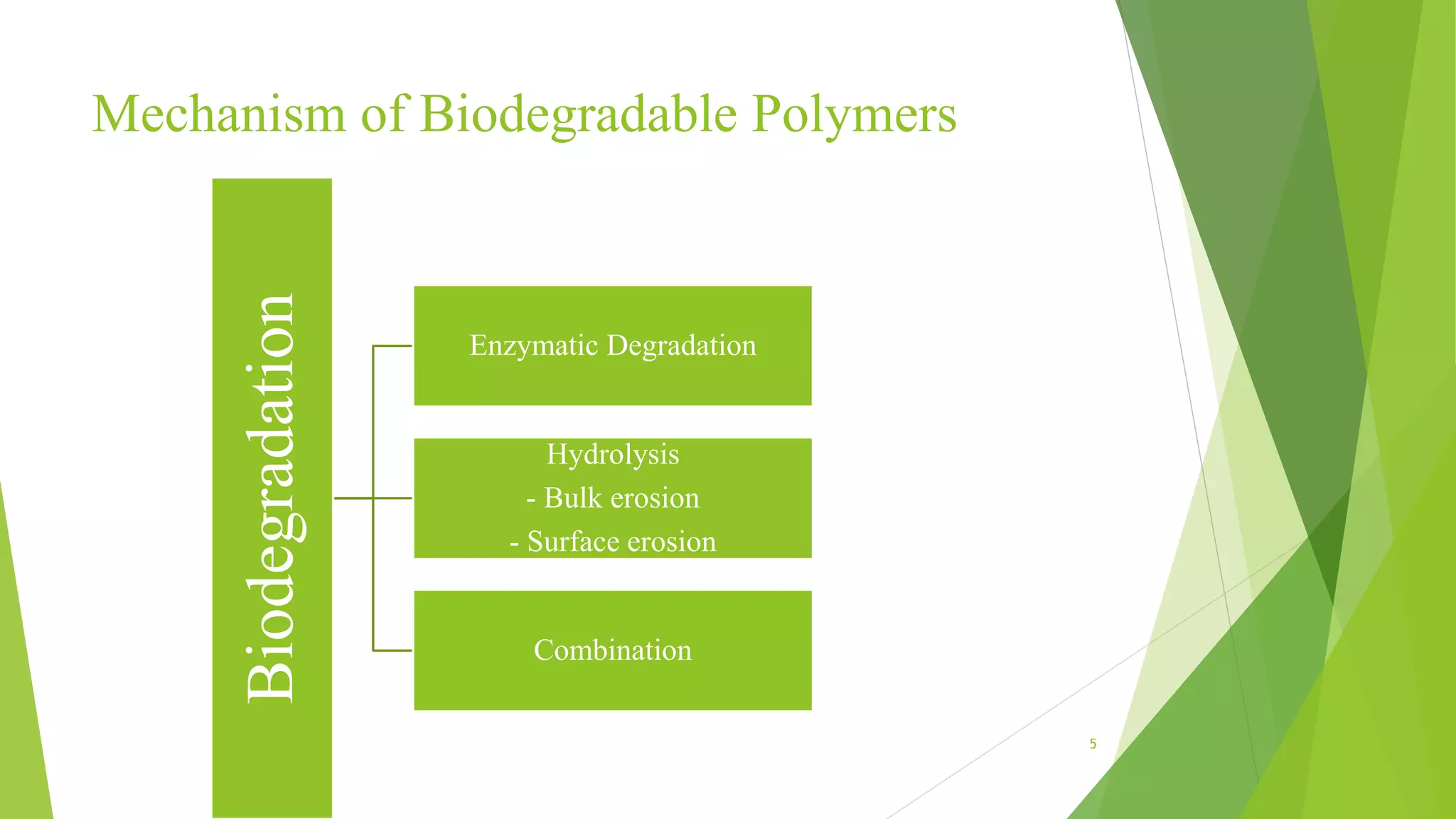
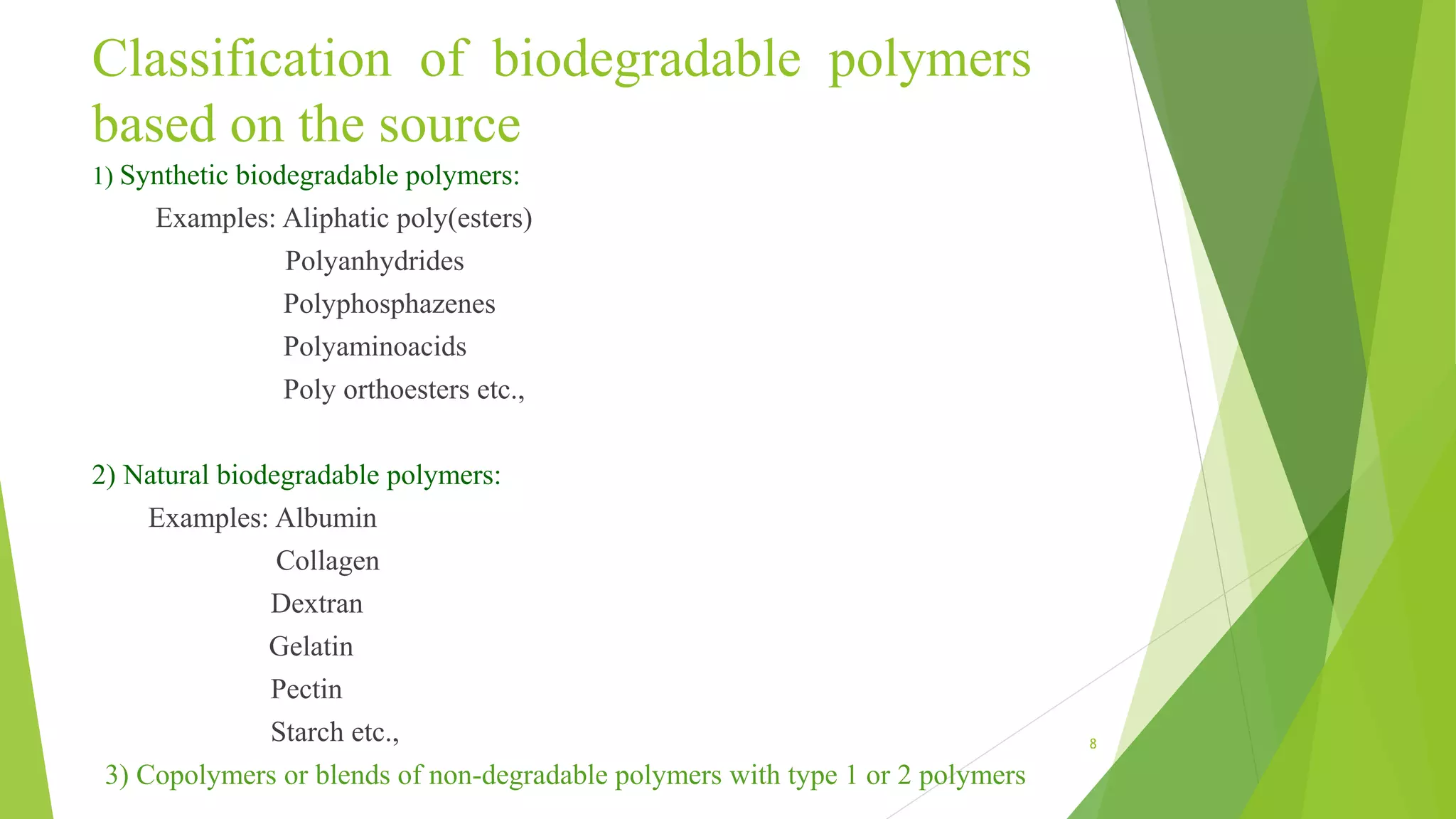
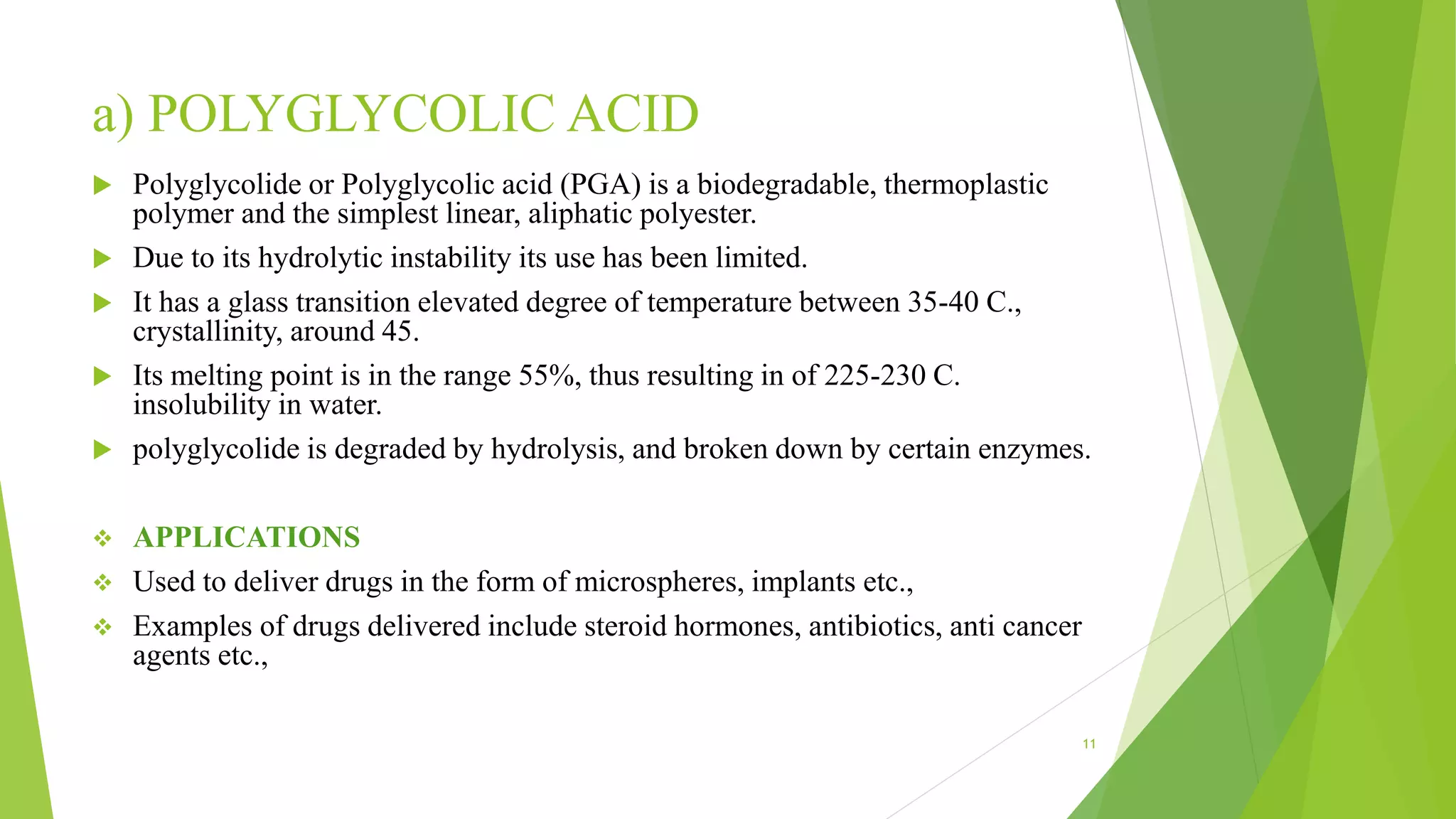
2) There are several types of biodegradable polymers including synthetic aliphatic polyesters like polylactic acid and polyglycolic acid, polyanhydrides, and natural polymers like collagen and gelatin. These polymers degrade via hydrolysis, surface erosion, or enzymatic degradation.
3) Biodegradable polymers have advantages for drug delivery such as localized and sustained release as well as reduced dosing requirements. However, challenges remain in controlling degradation rates and maintaining drug stability