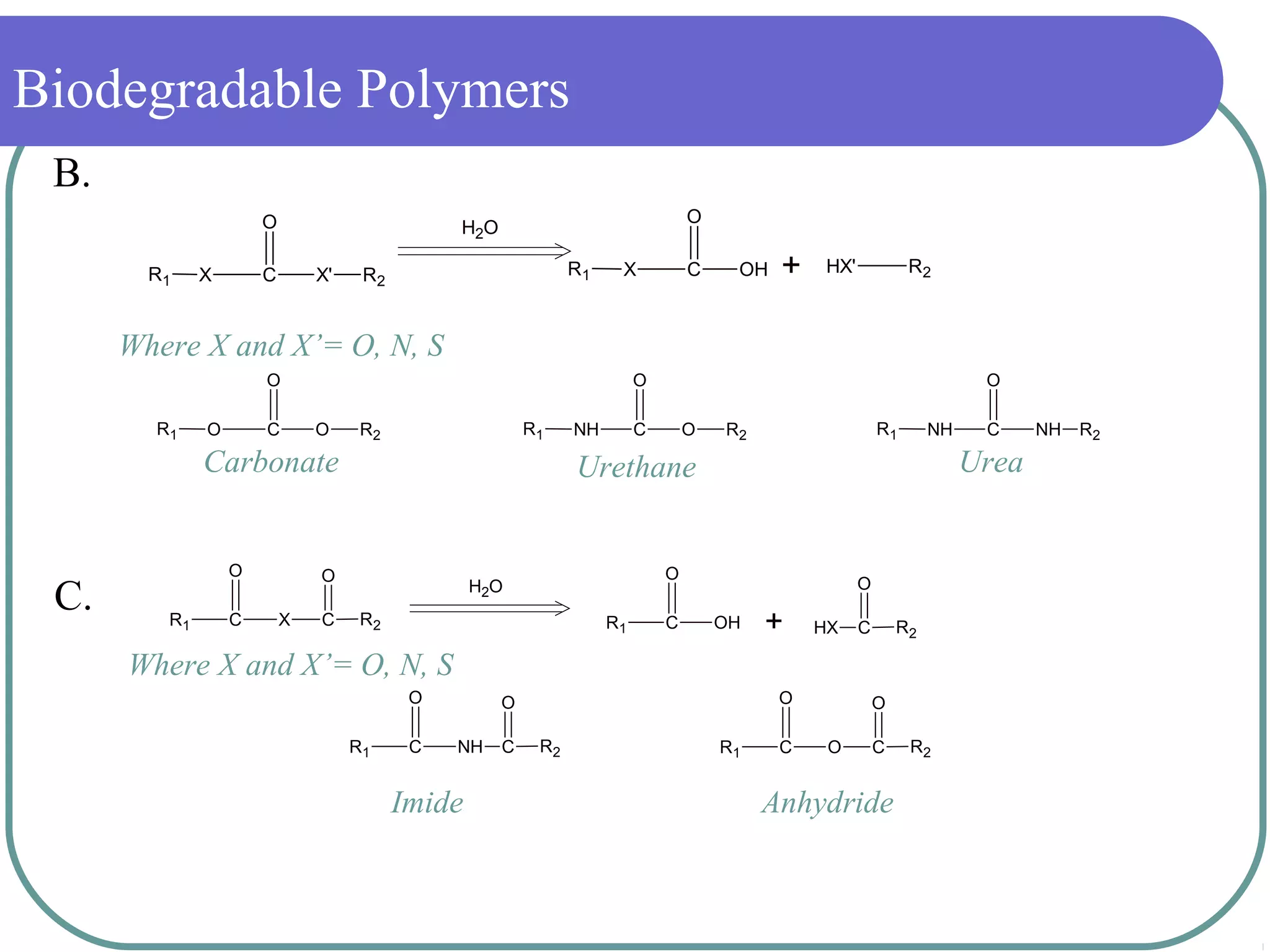
The document discusses biodegradable polymers and their medical applications. It defines polymer degradation as the process where properties and performance deteriorate over time. For medical uses, biodegradable polymers are desirable as they do not require removal surgery and can provide controlled drug delivery. Common biodegradable polymers discussed include PLA, PGA and PLGA. The mechanisms of degradation include hydrolysis and enzymatic processes. Medical applications discussed include sutures, implants, stents and drug delivery systems.