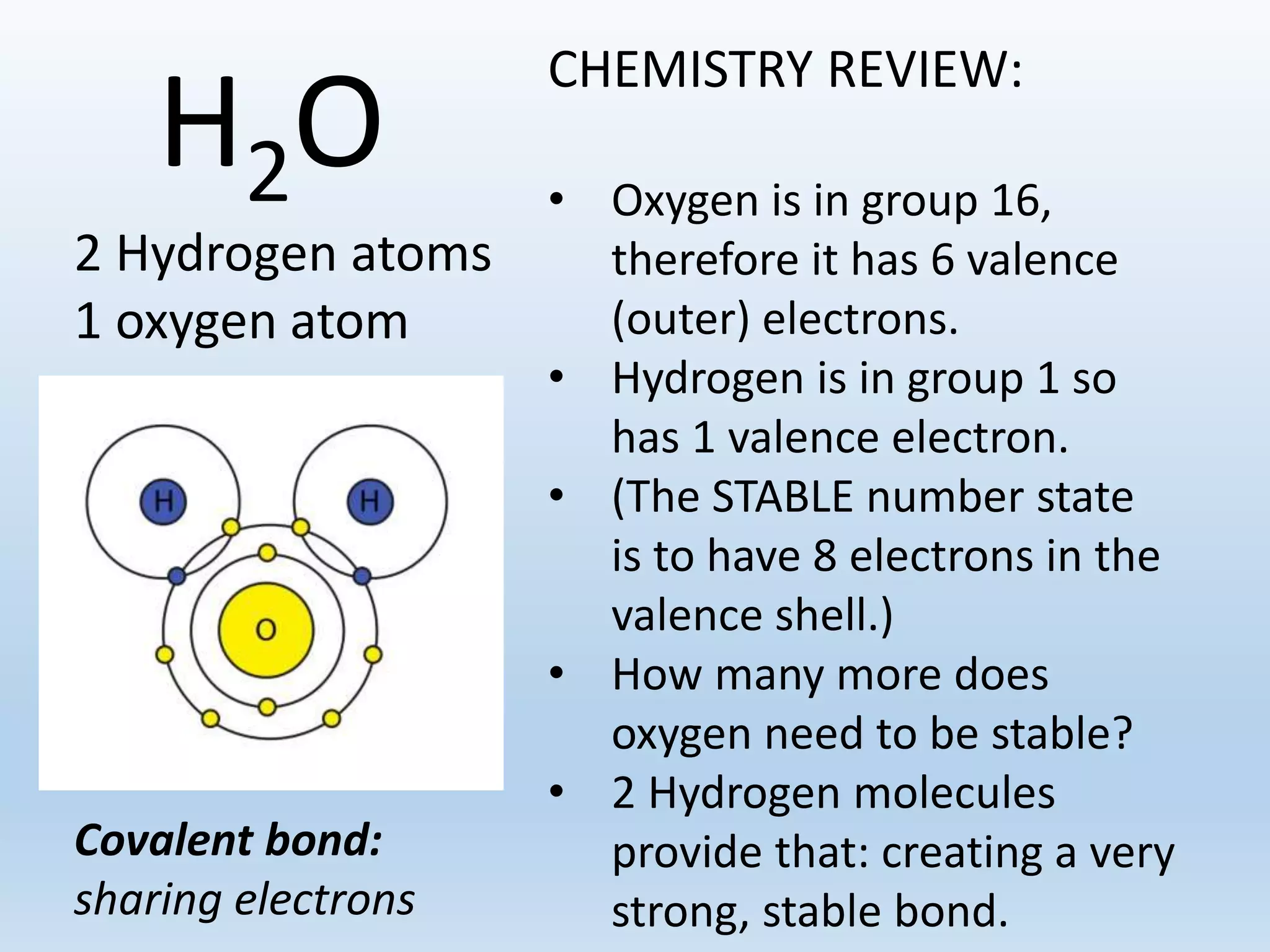
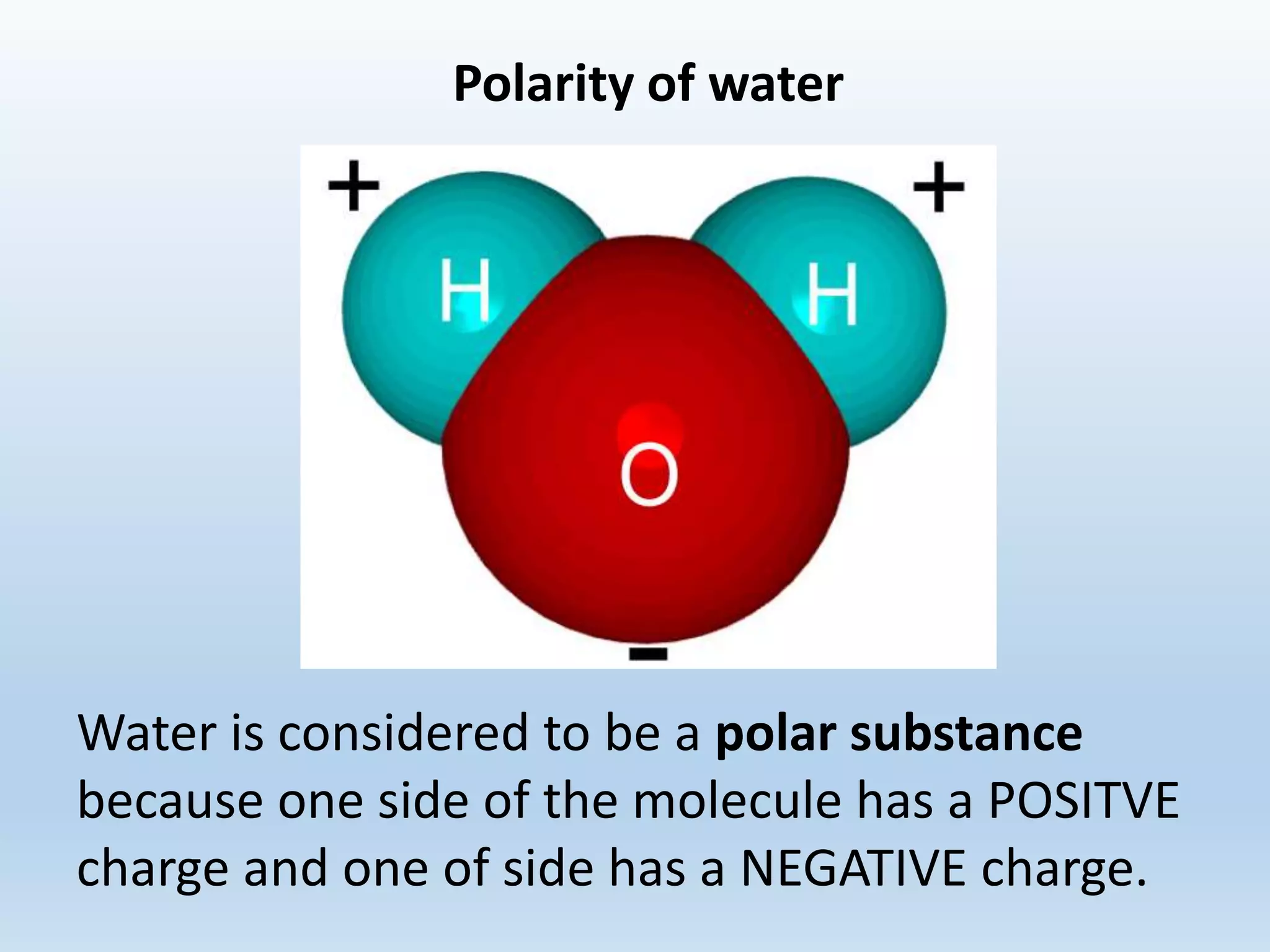
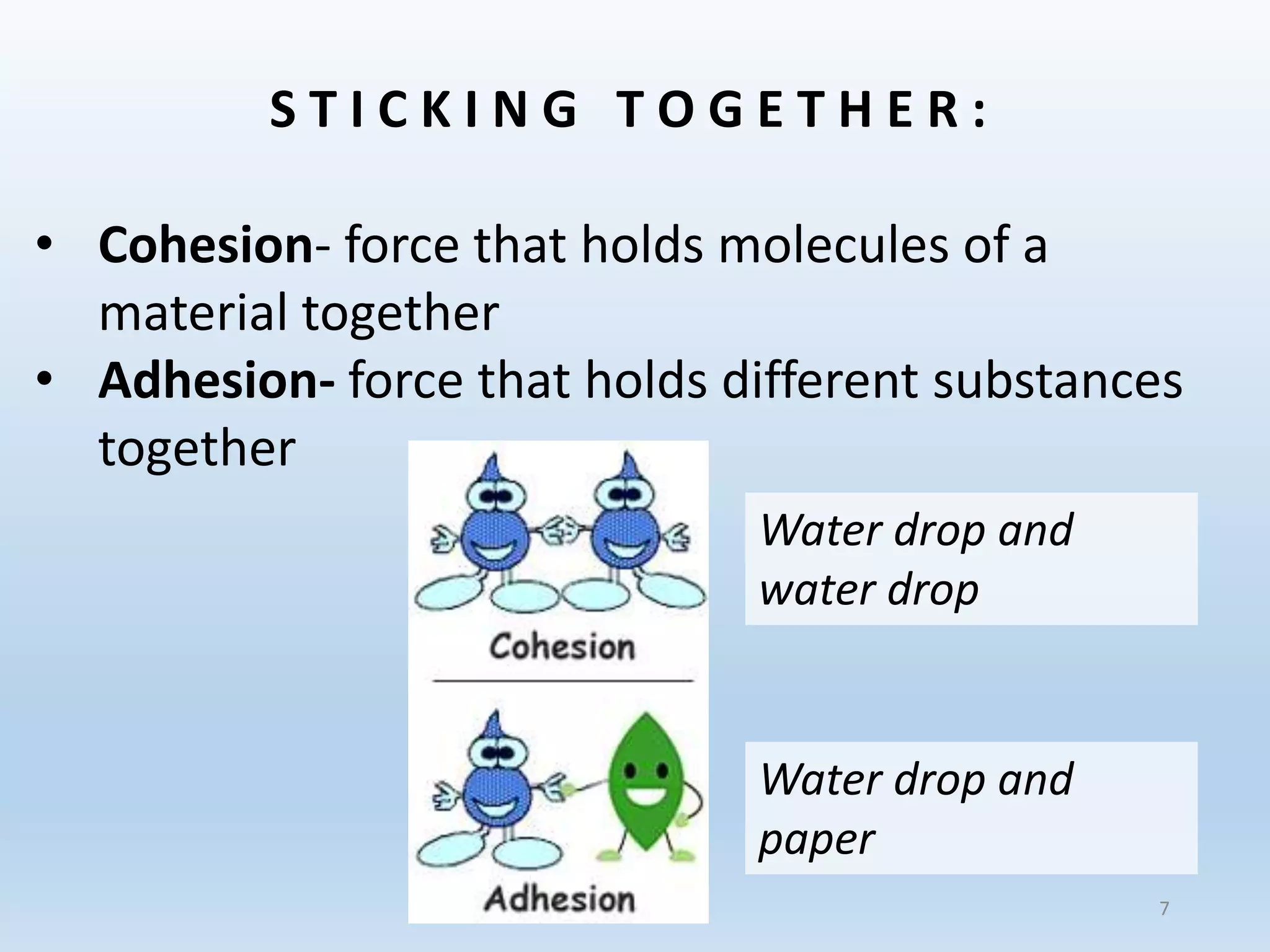
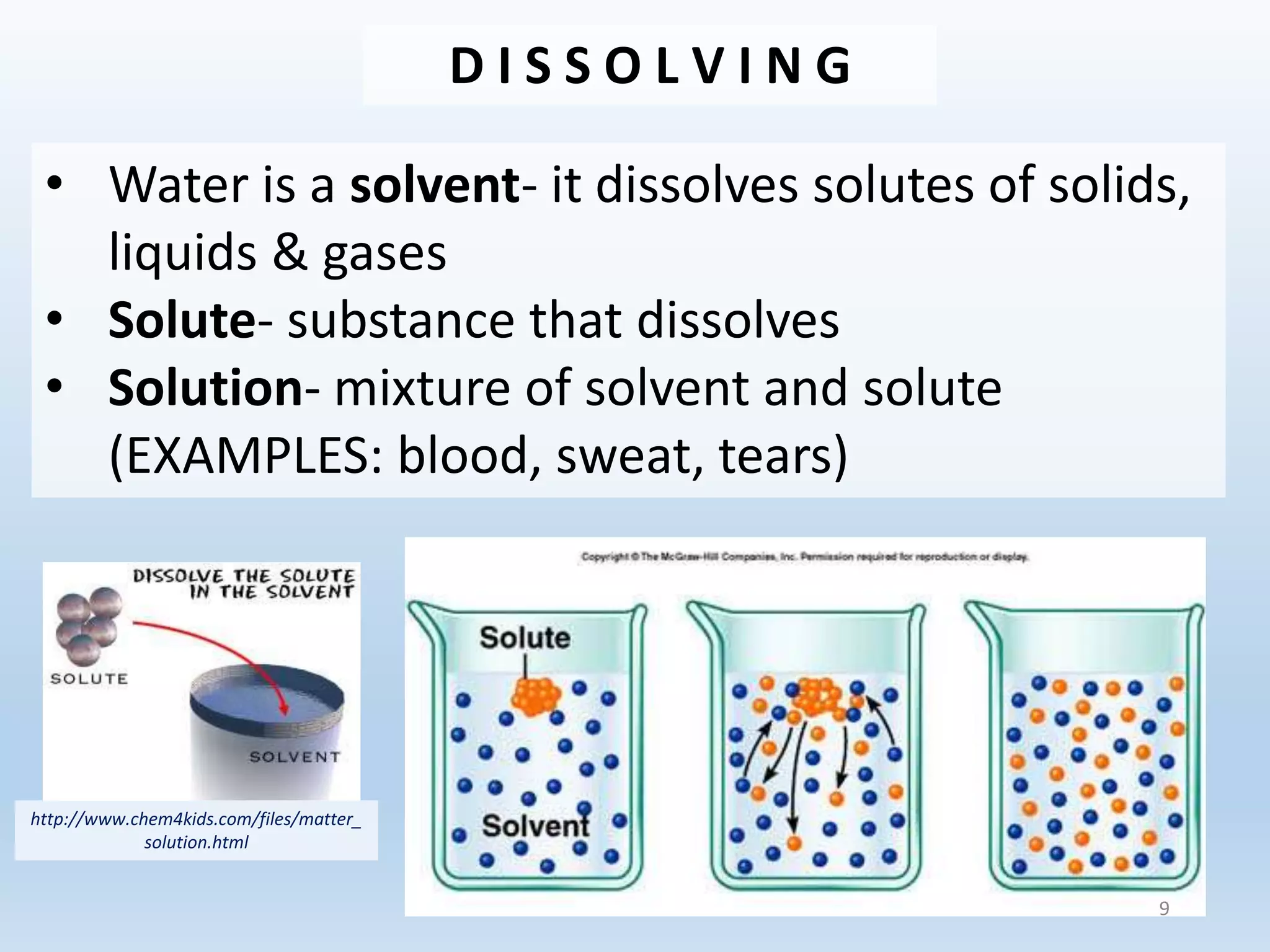
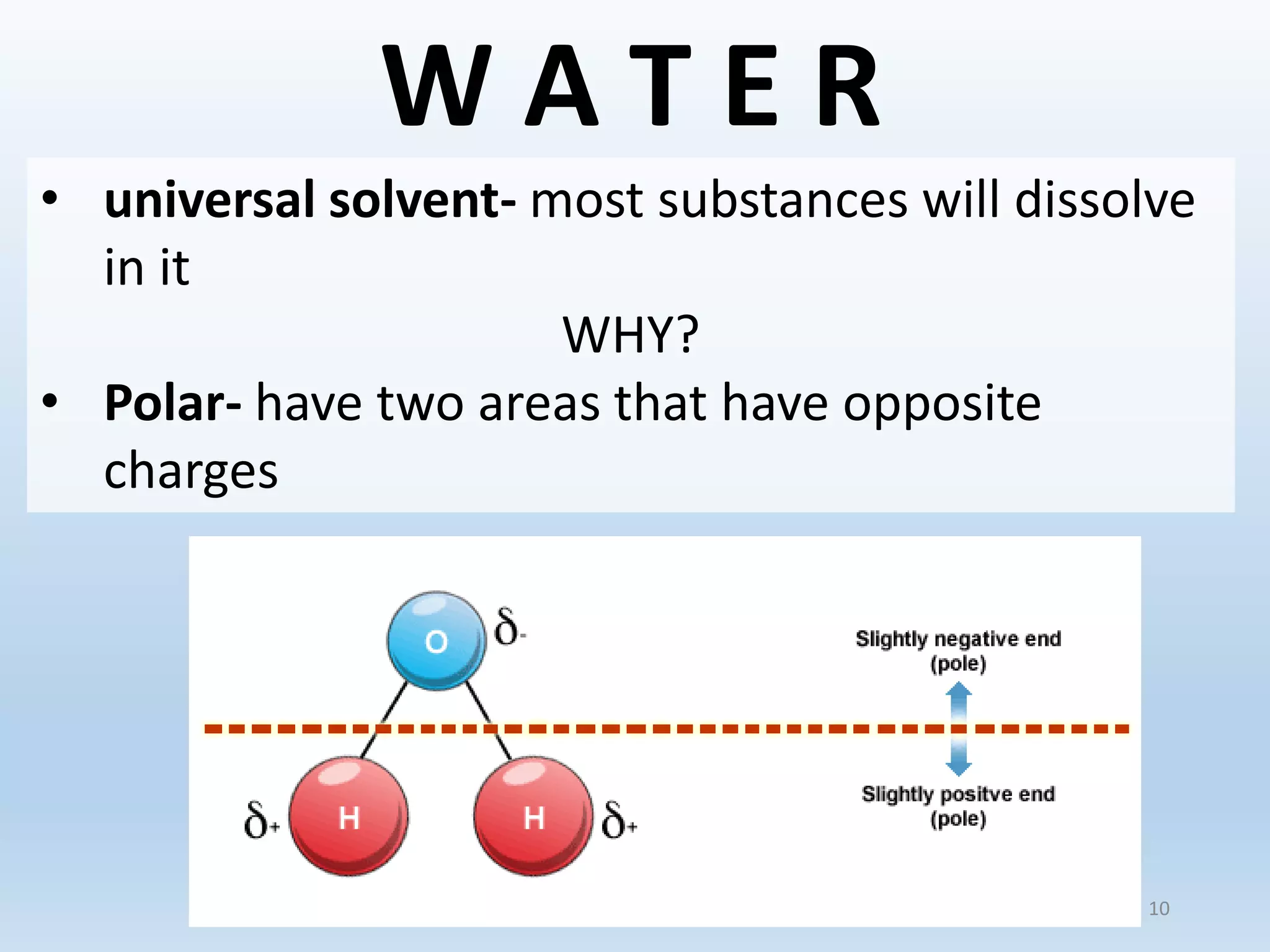
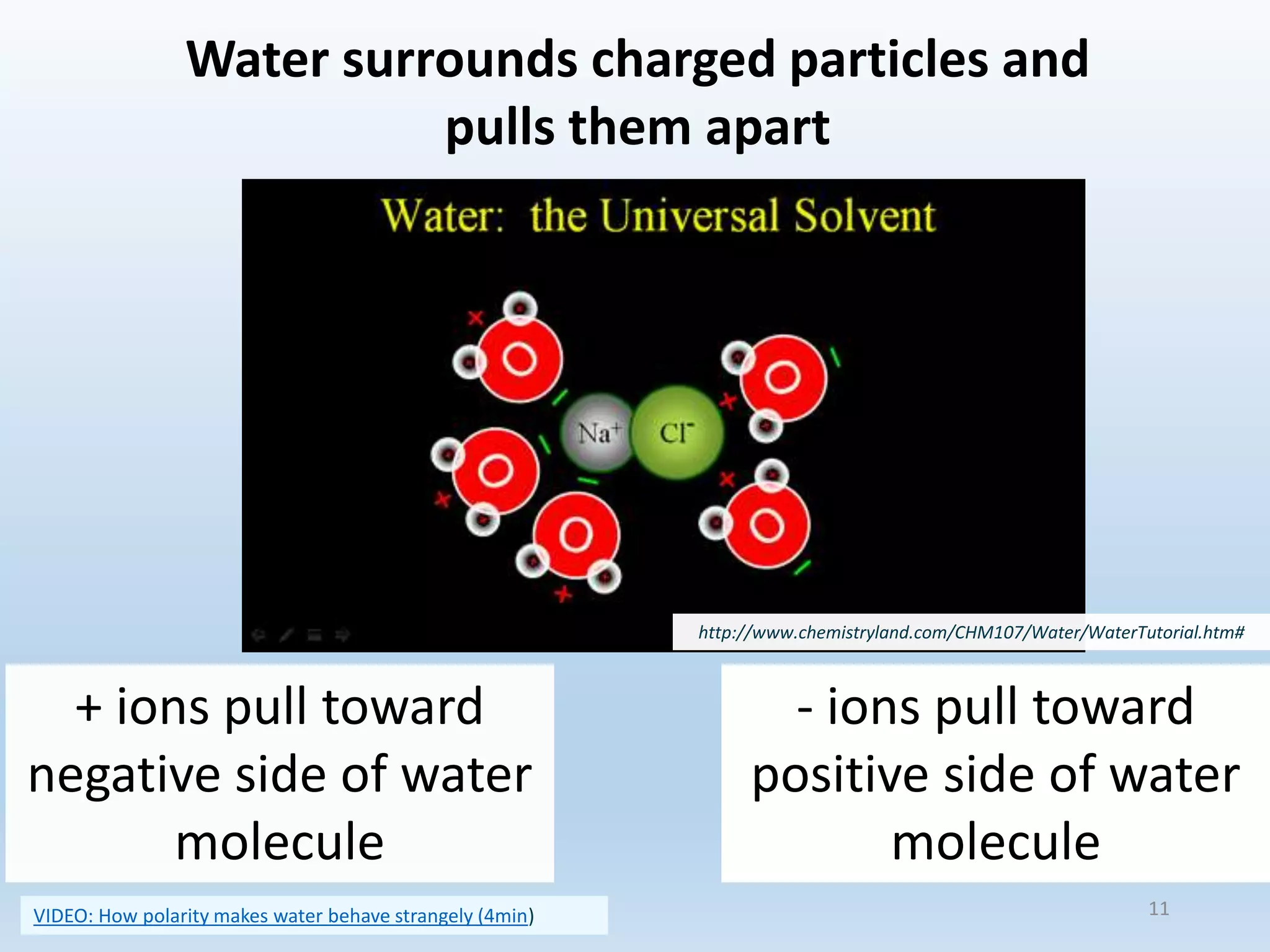
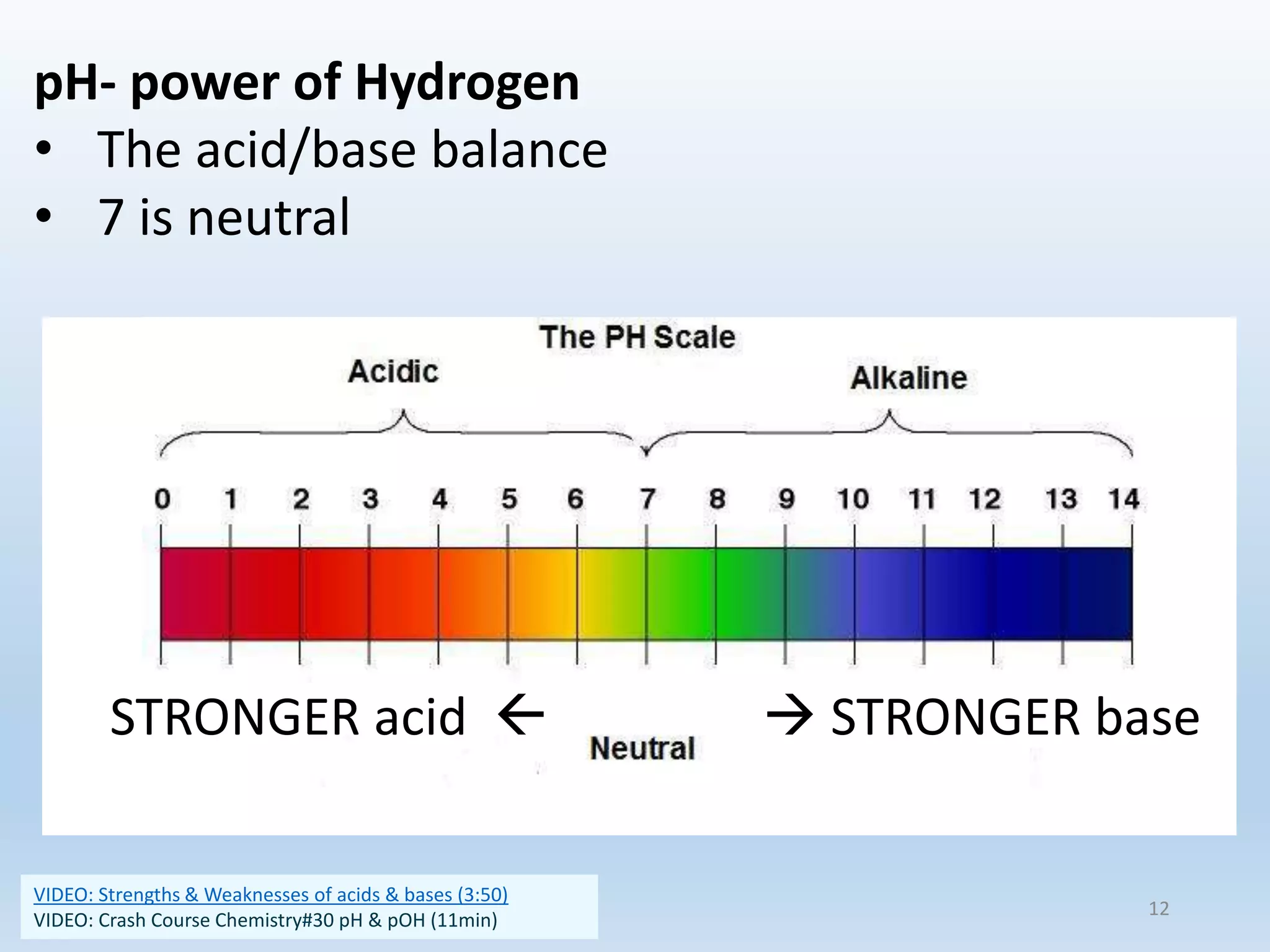
This document provides an overview of the importance and properties of water. It discusses how water is essential to many bodily processes since humans are about 60% water. Water is able to carry out these functions because of its unique molecular structure and polarity. The polarity of water allows it to dissolve many other substances and interact with ions through hydrogen bonding. These cohesive and adhesive properties give water its high surface tension and ability to act as a solvent. The document also briefly covers pH, acids, bases, and buffers.