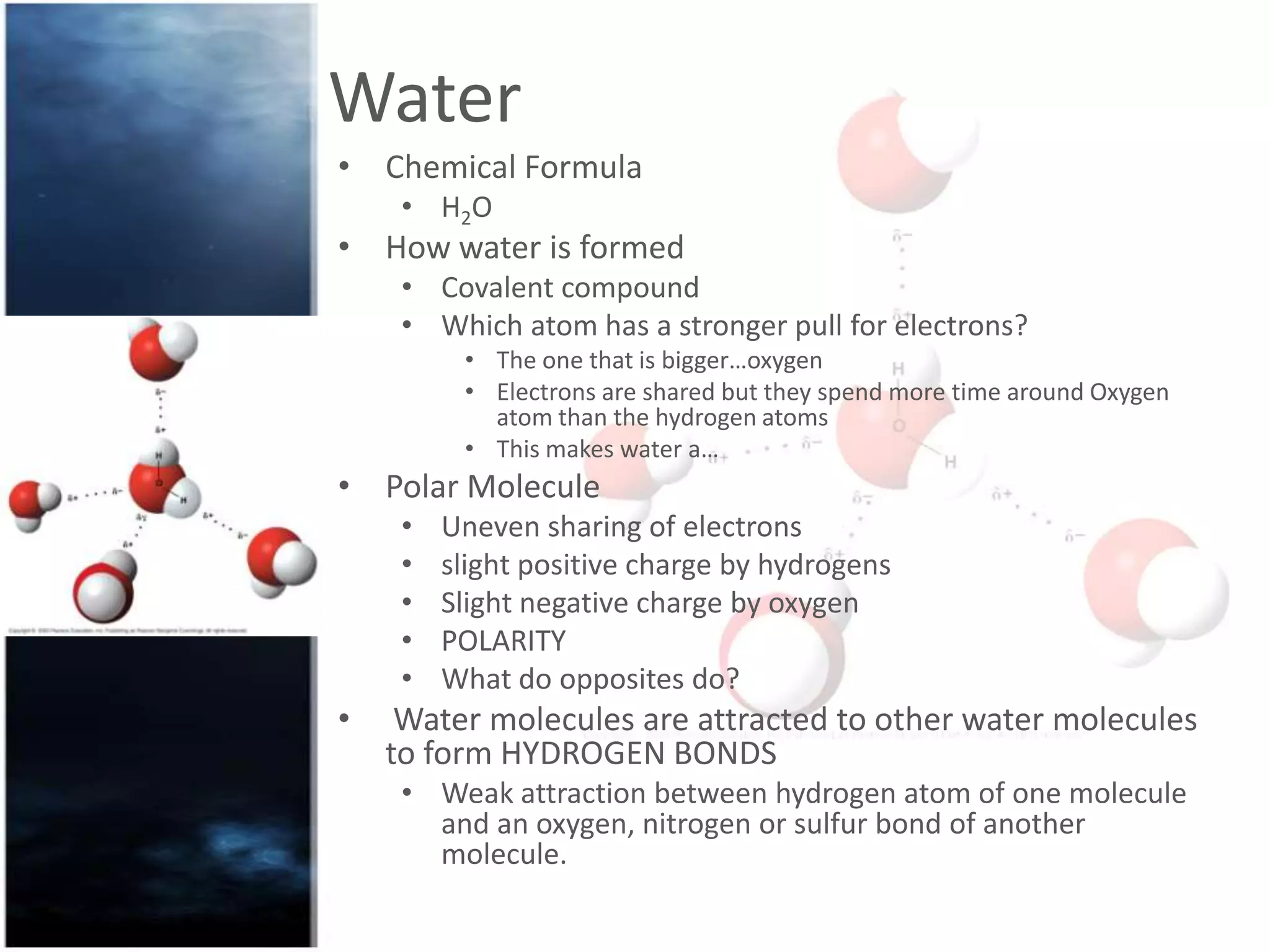
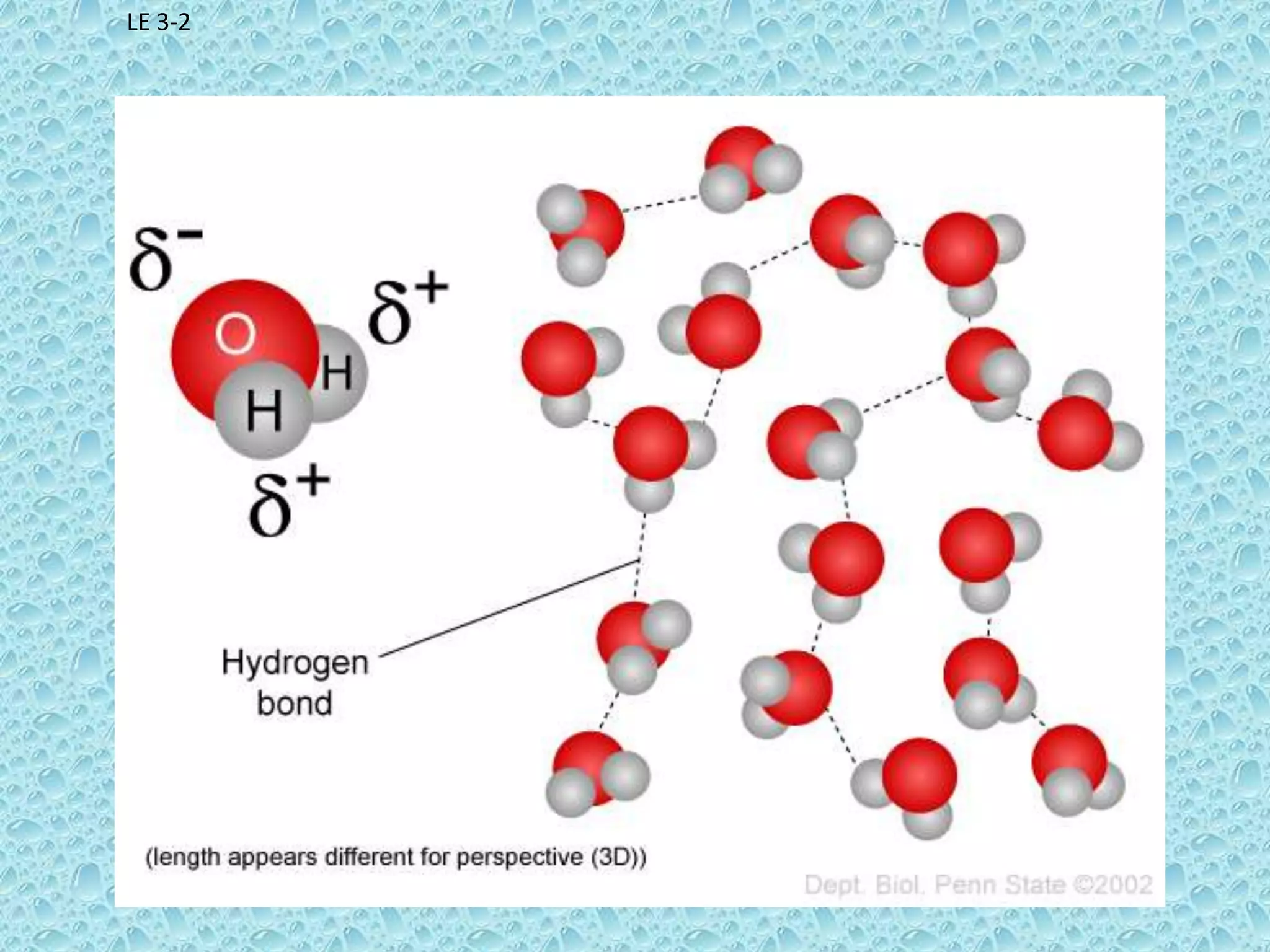
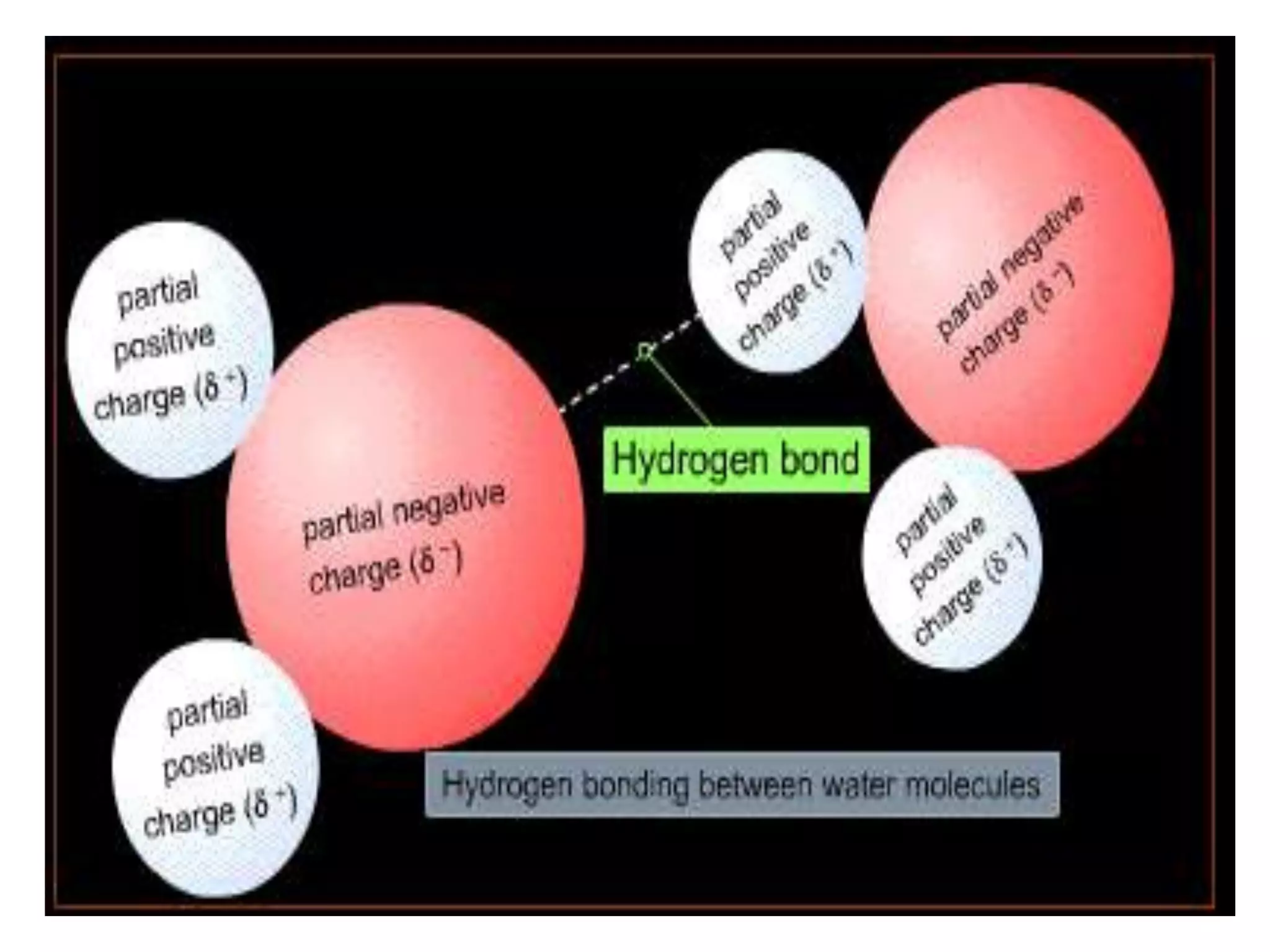
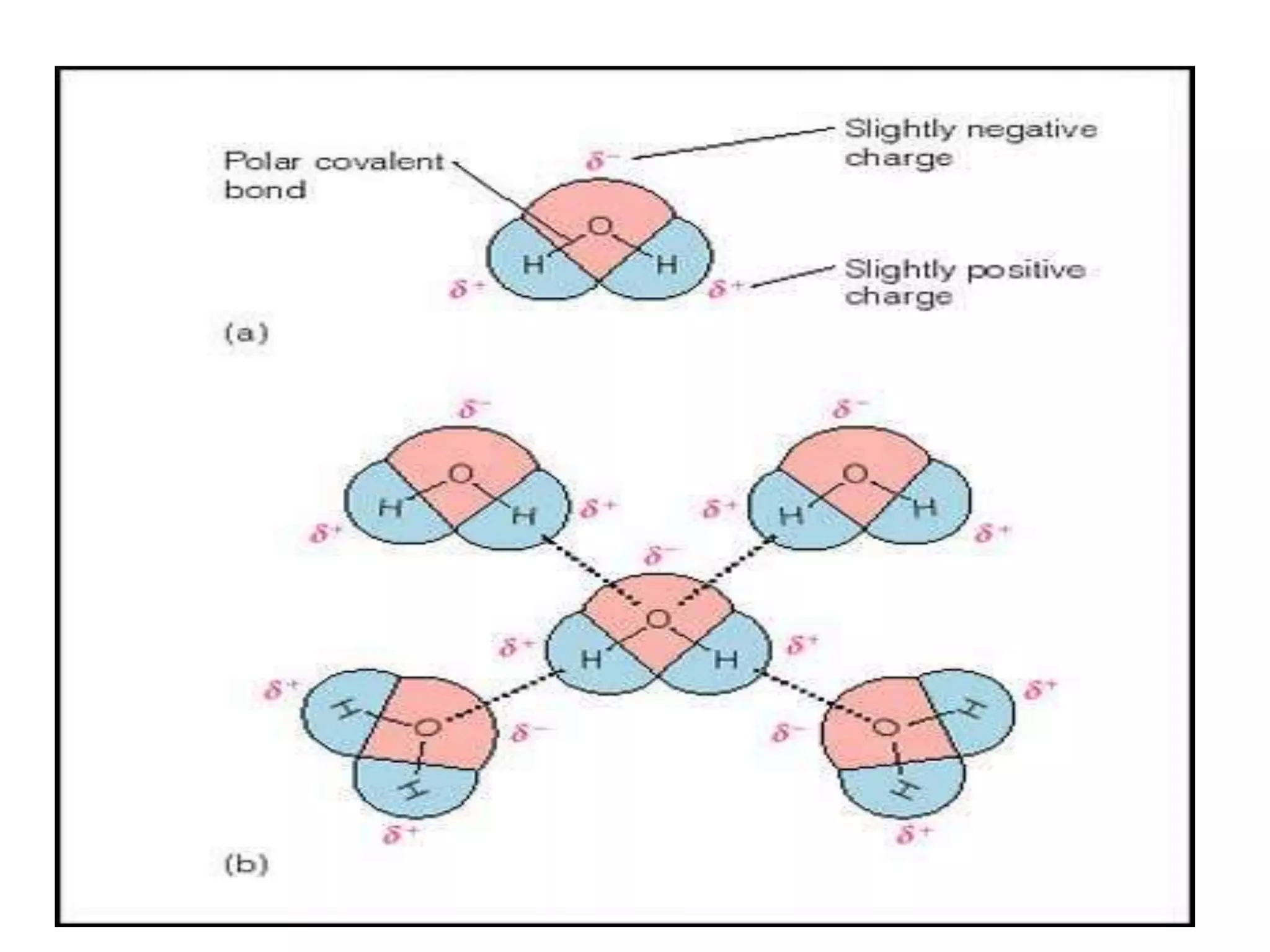
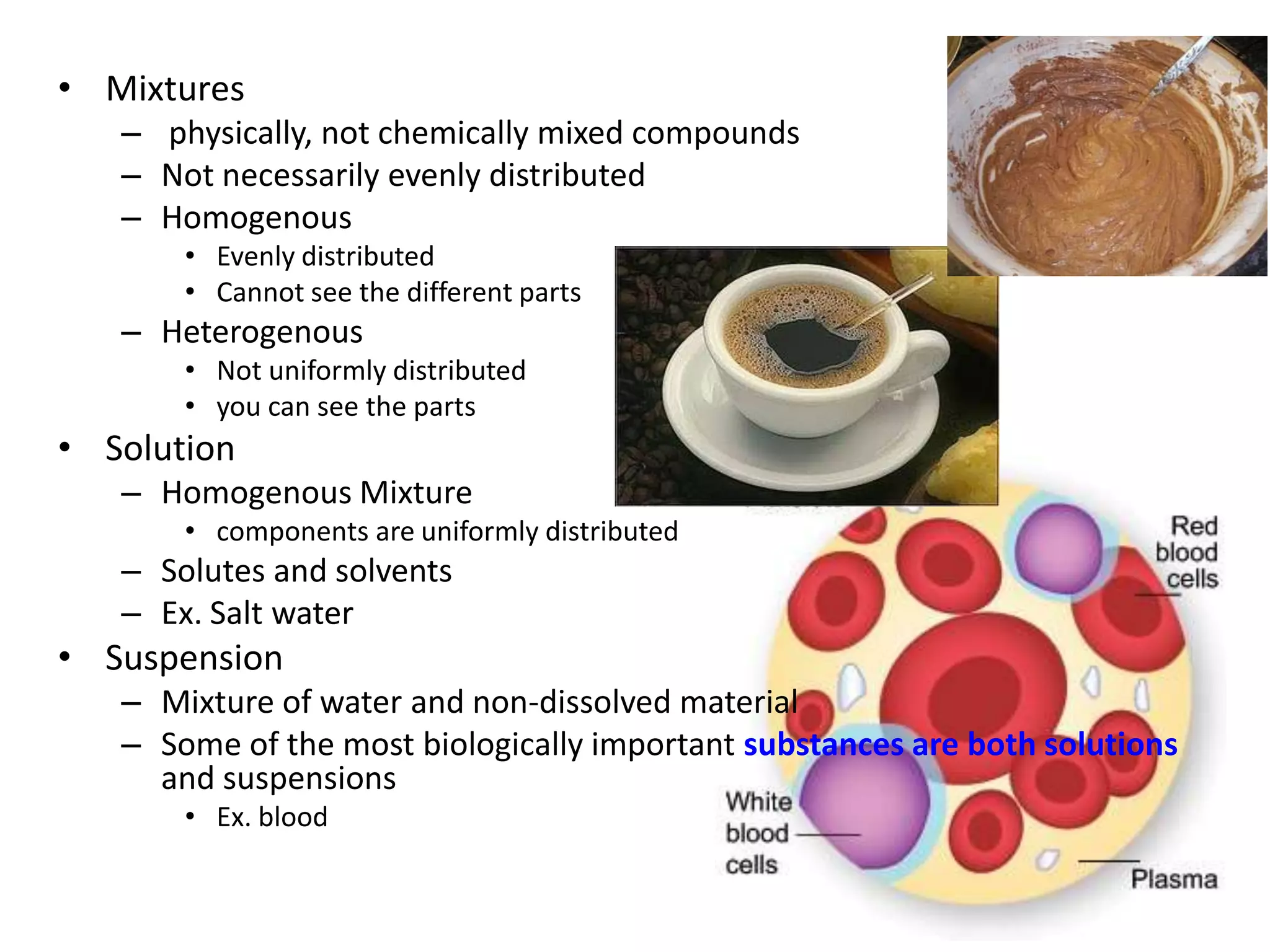
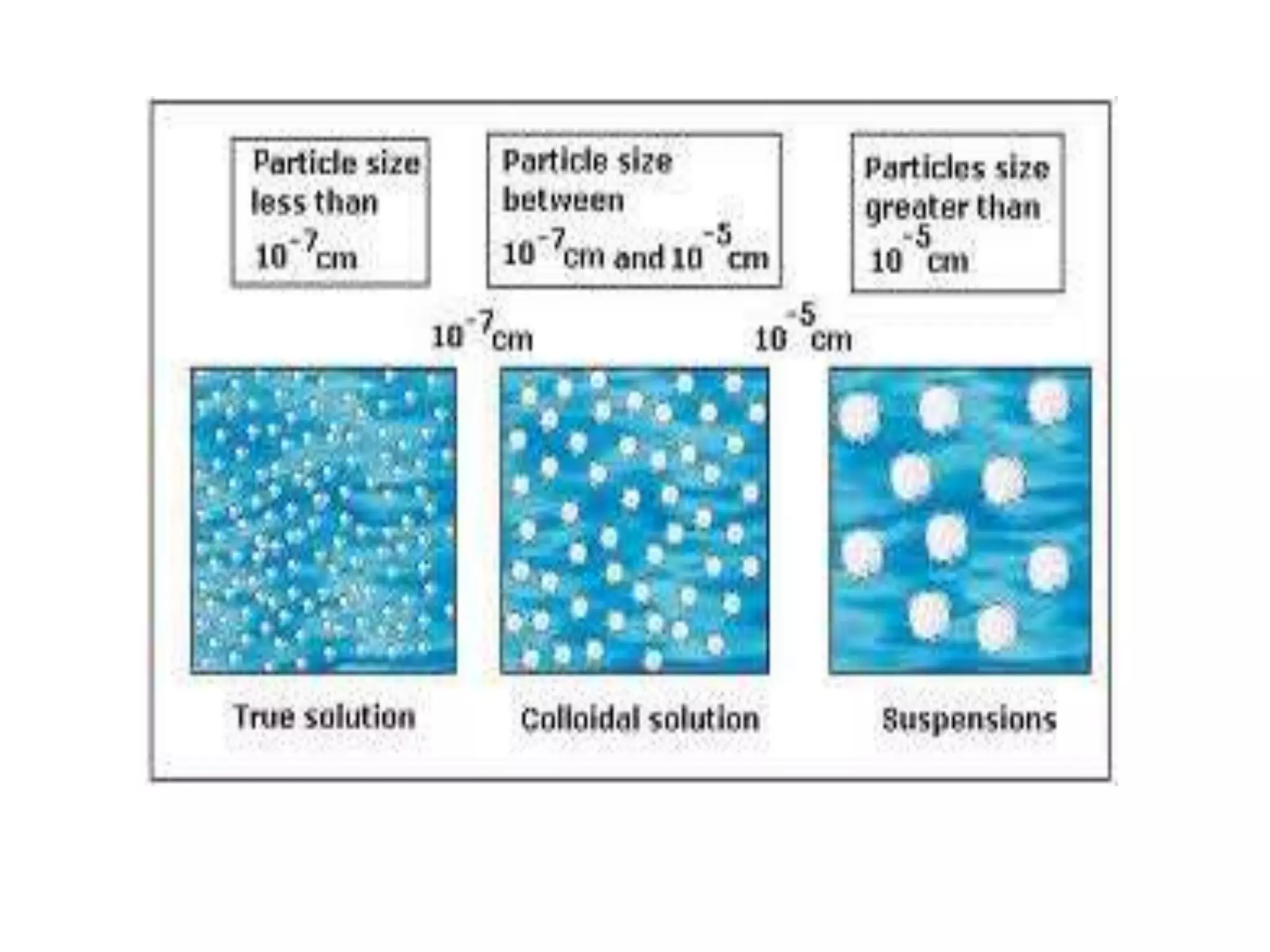
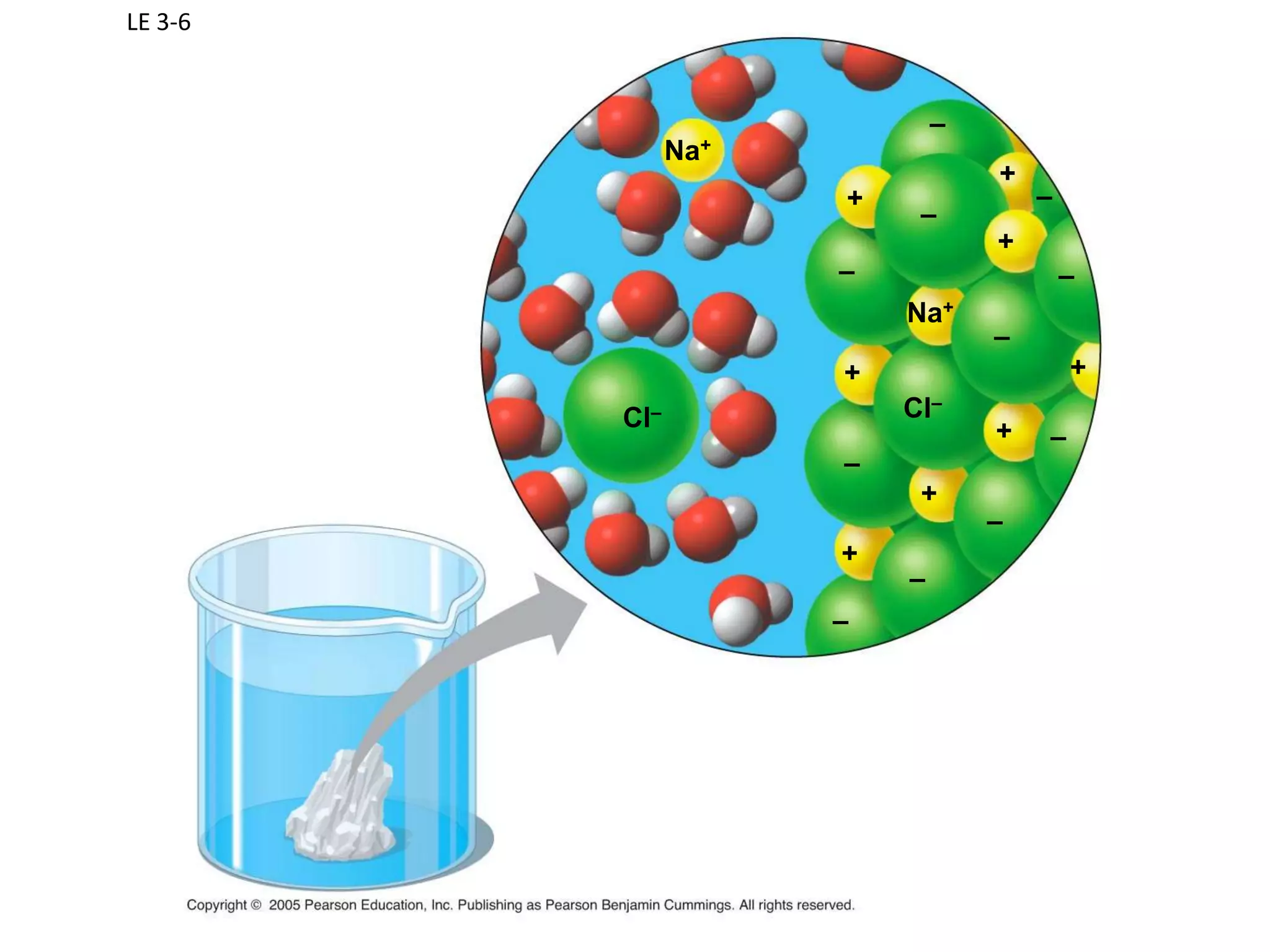
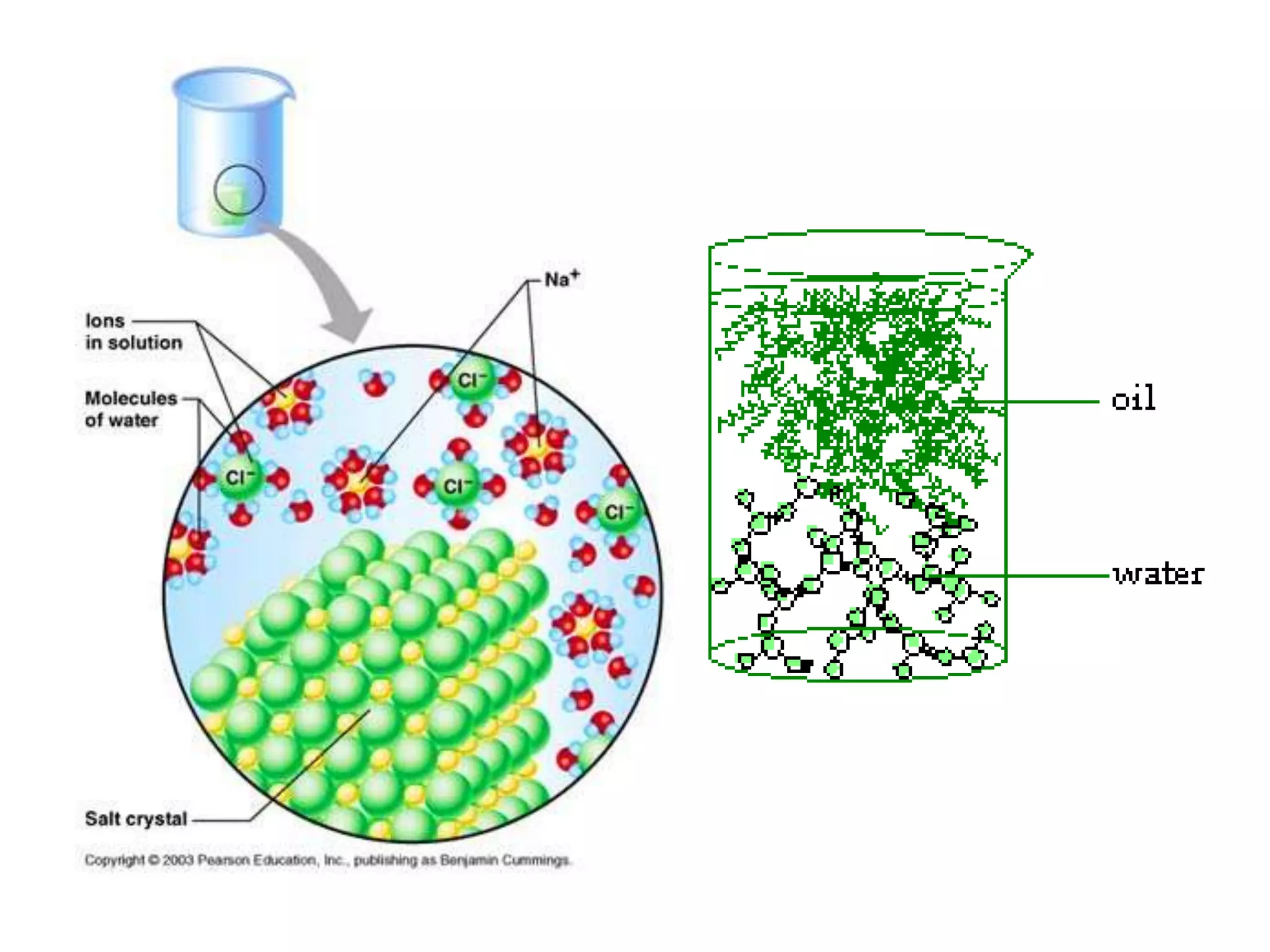
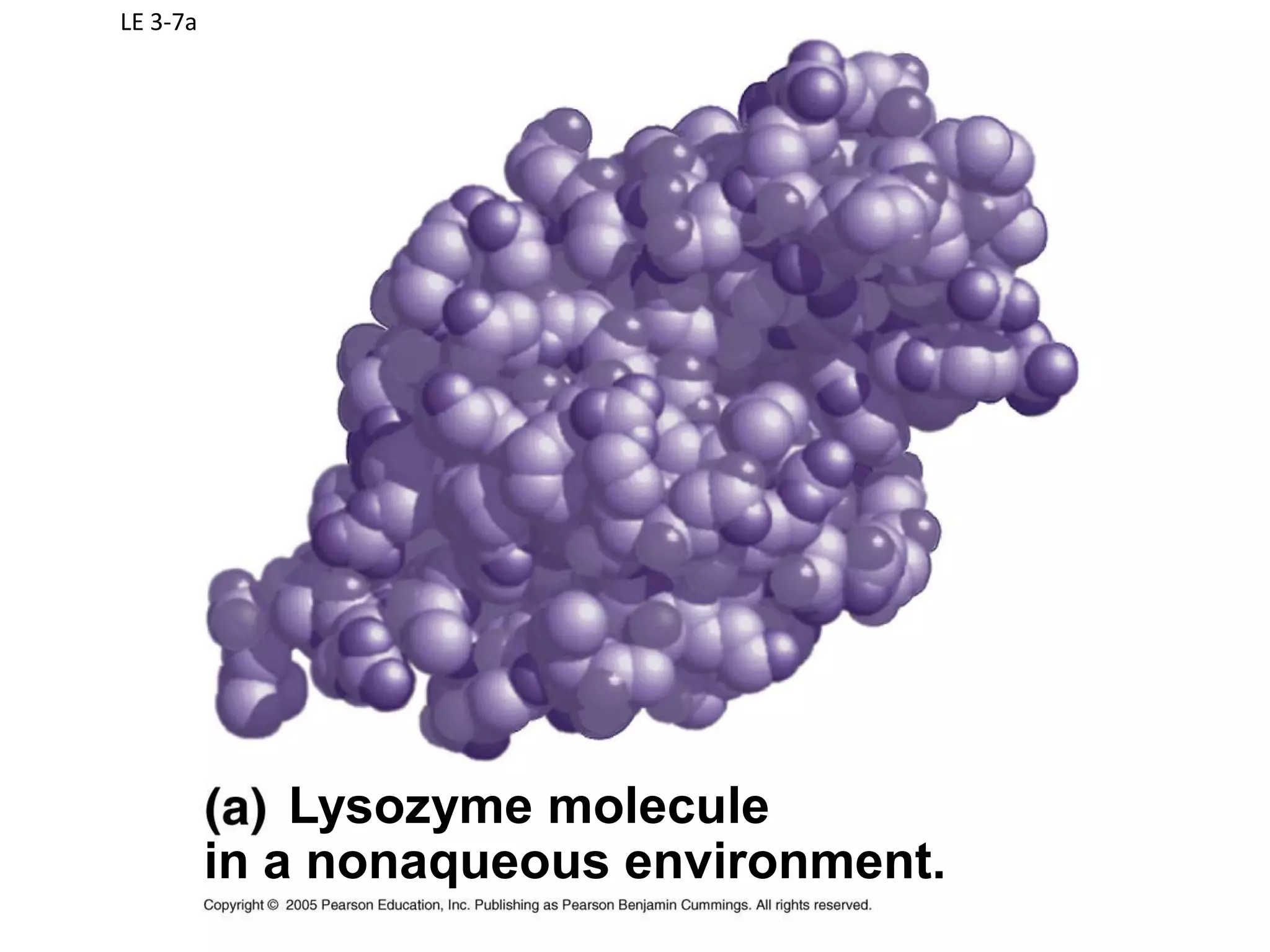
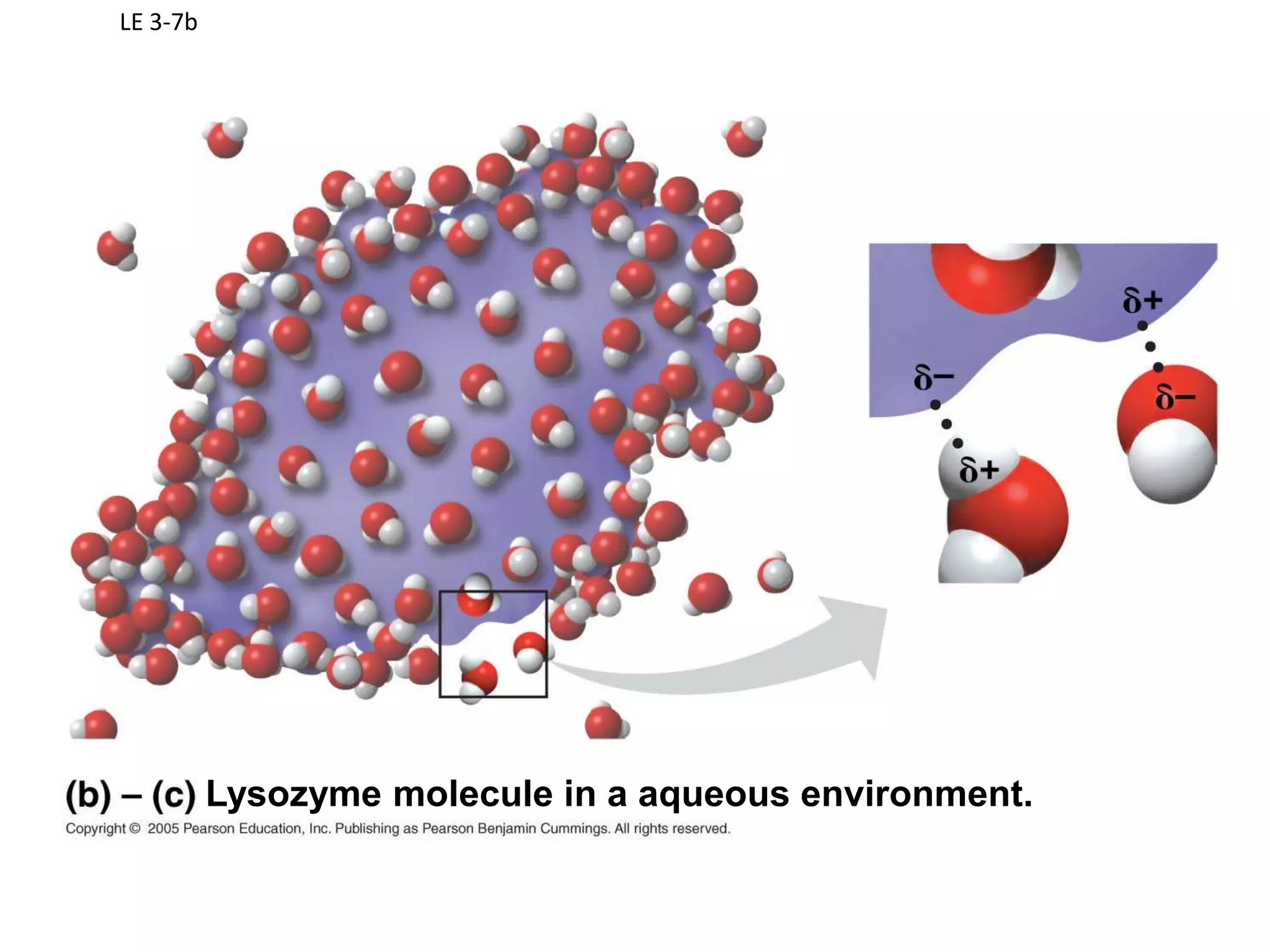
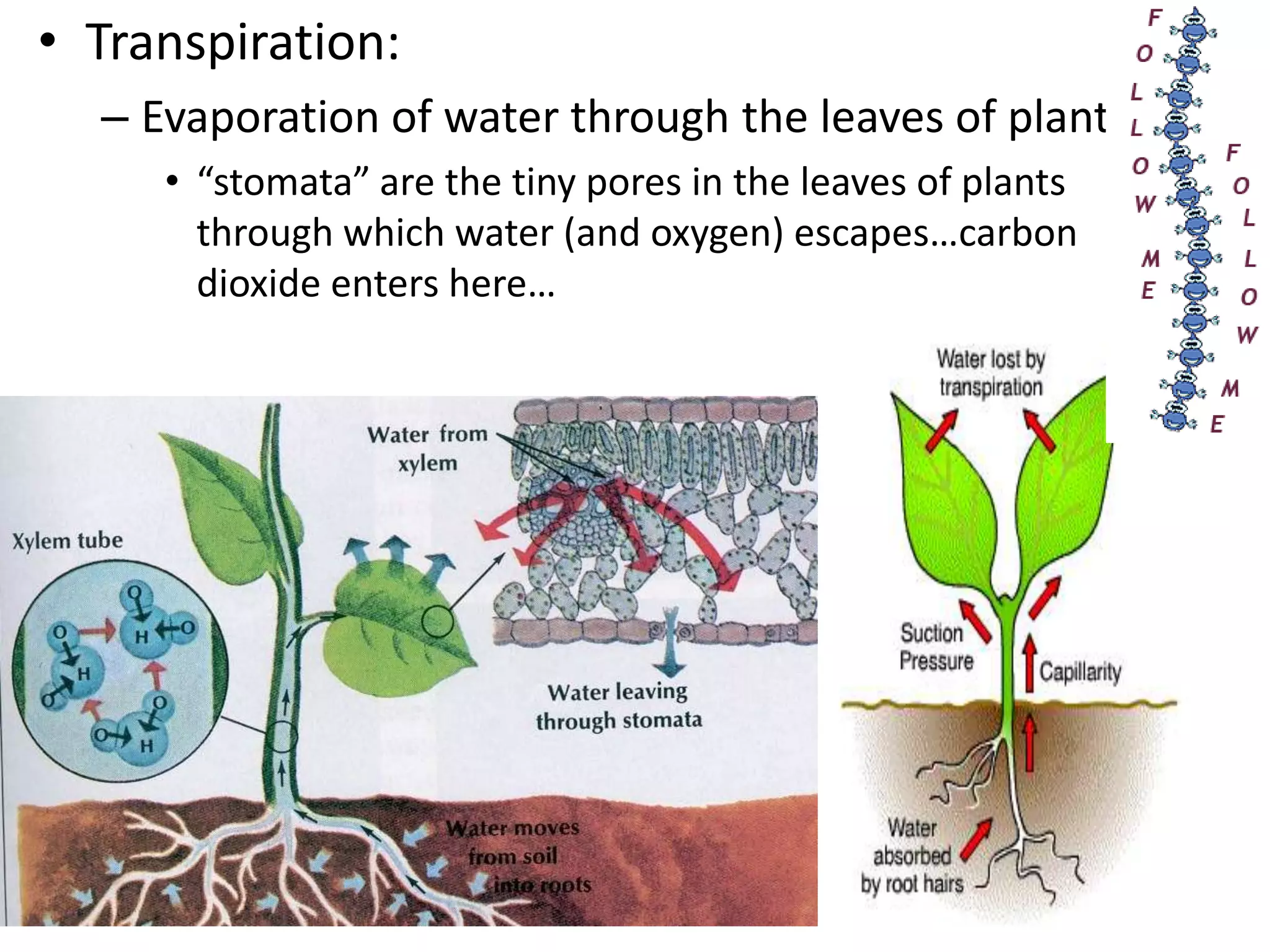
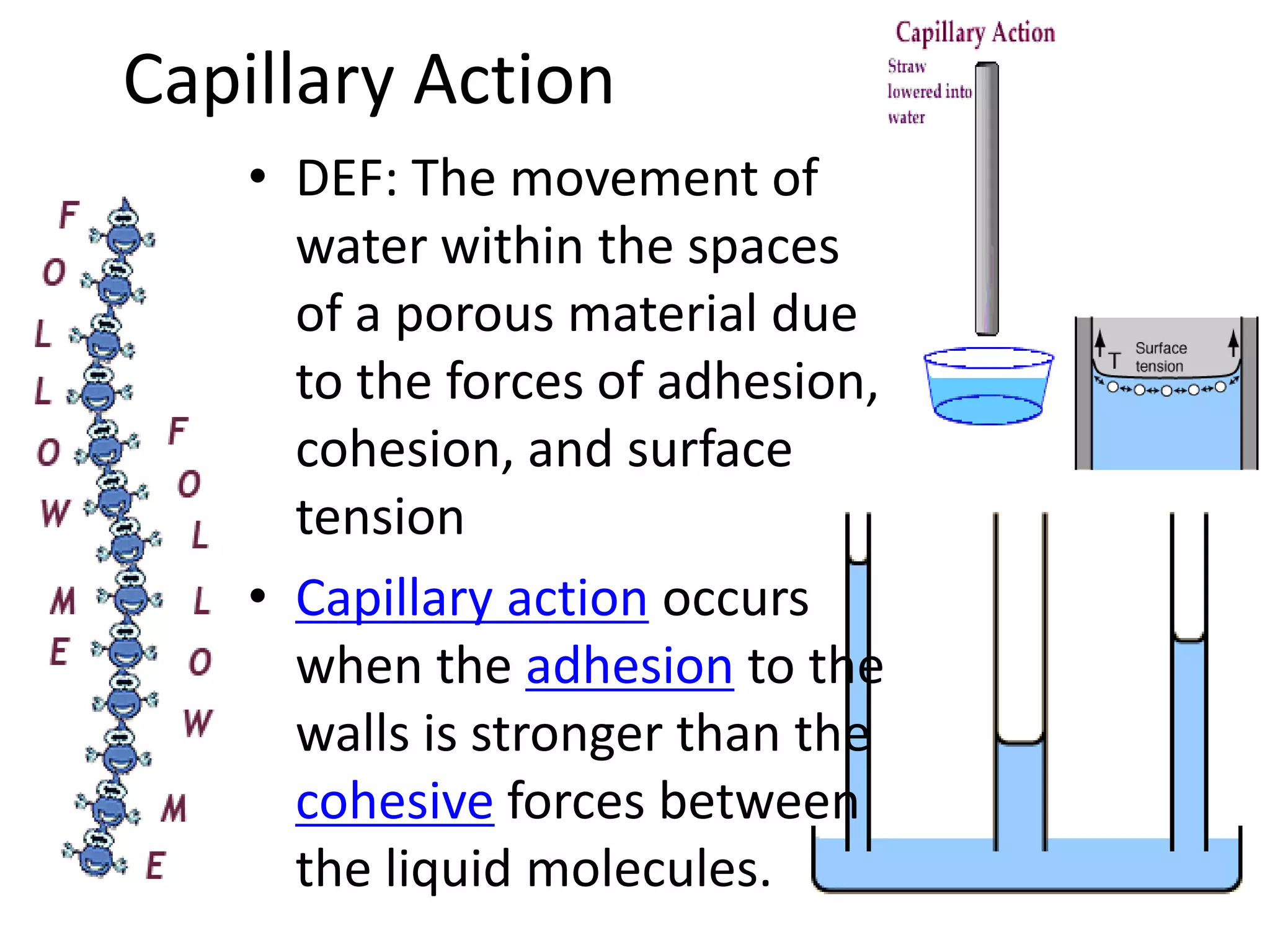
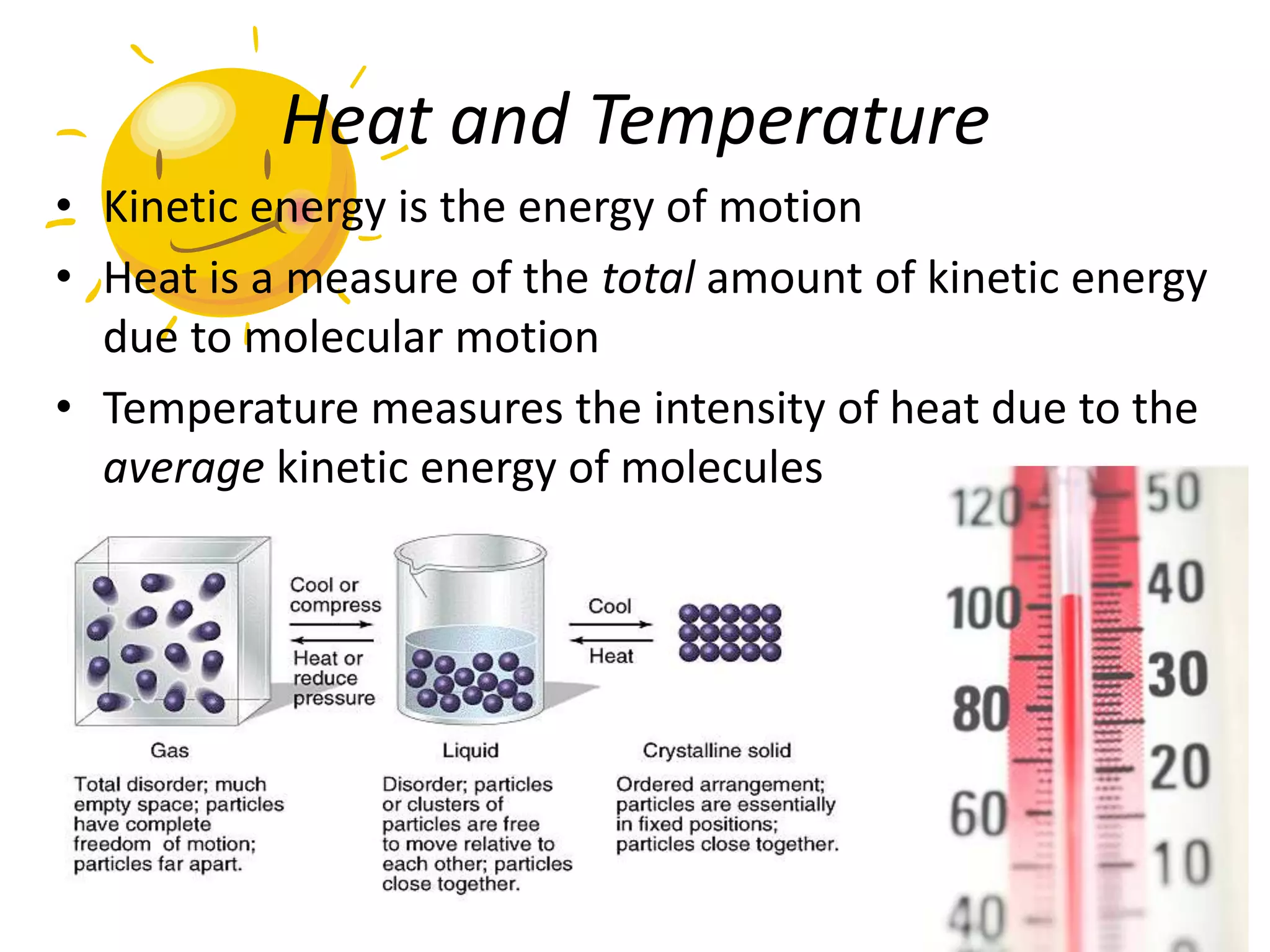
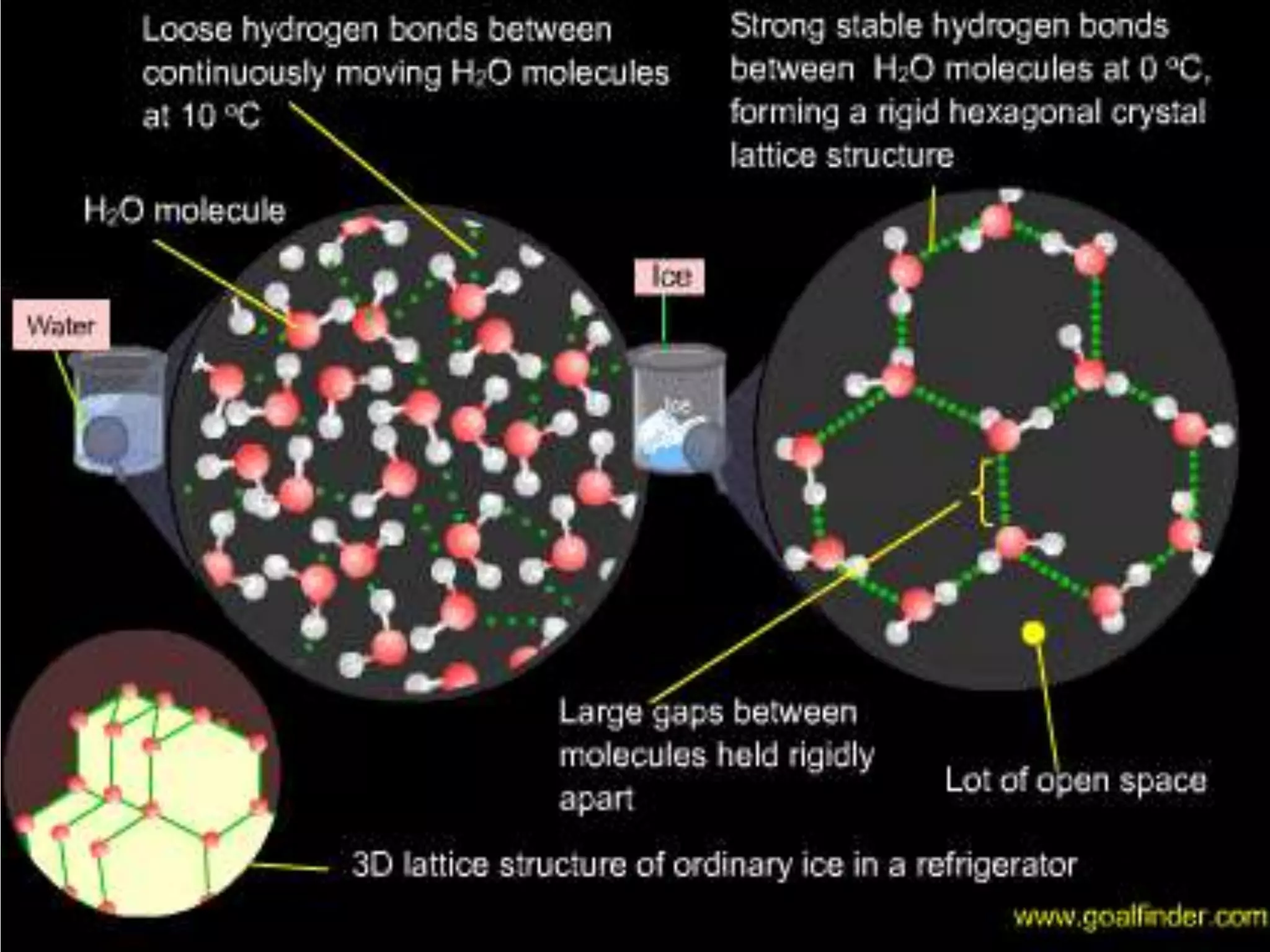
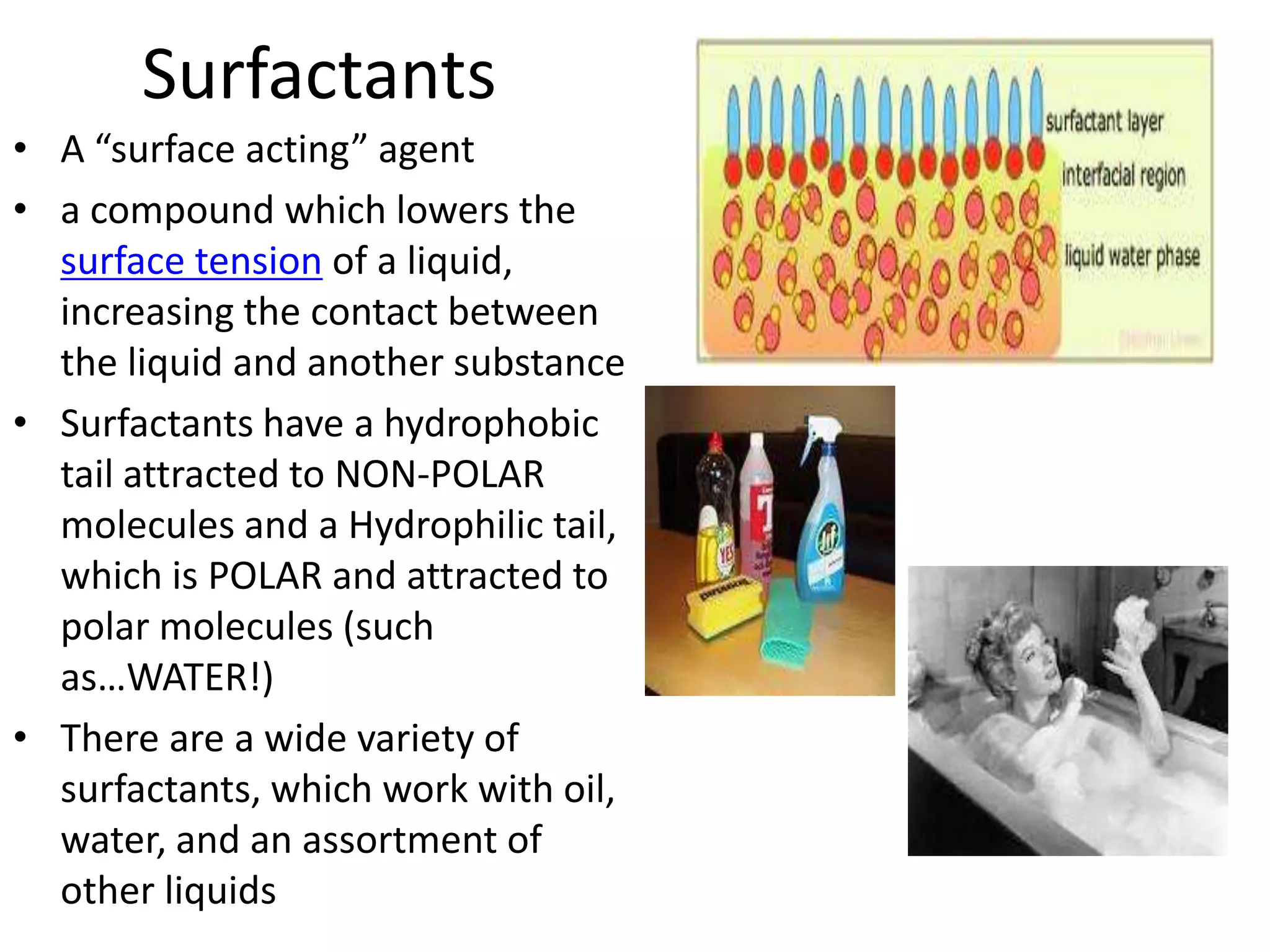
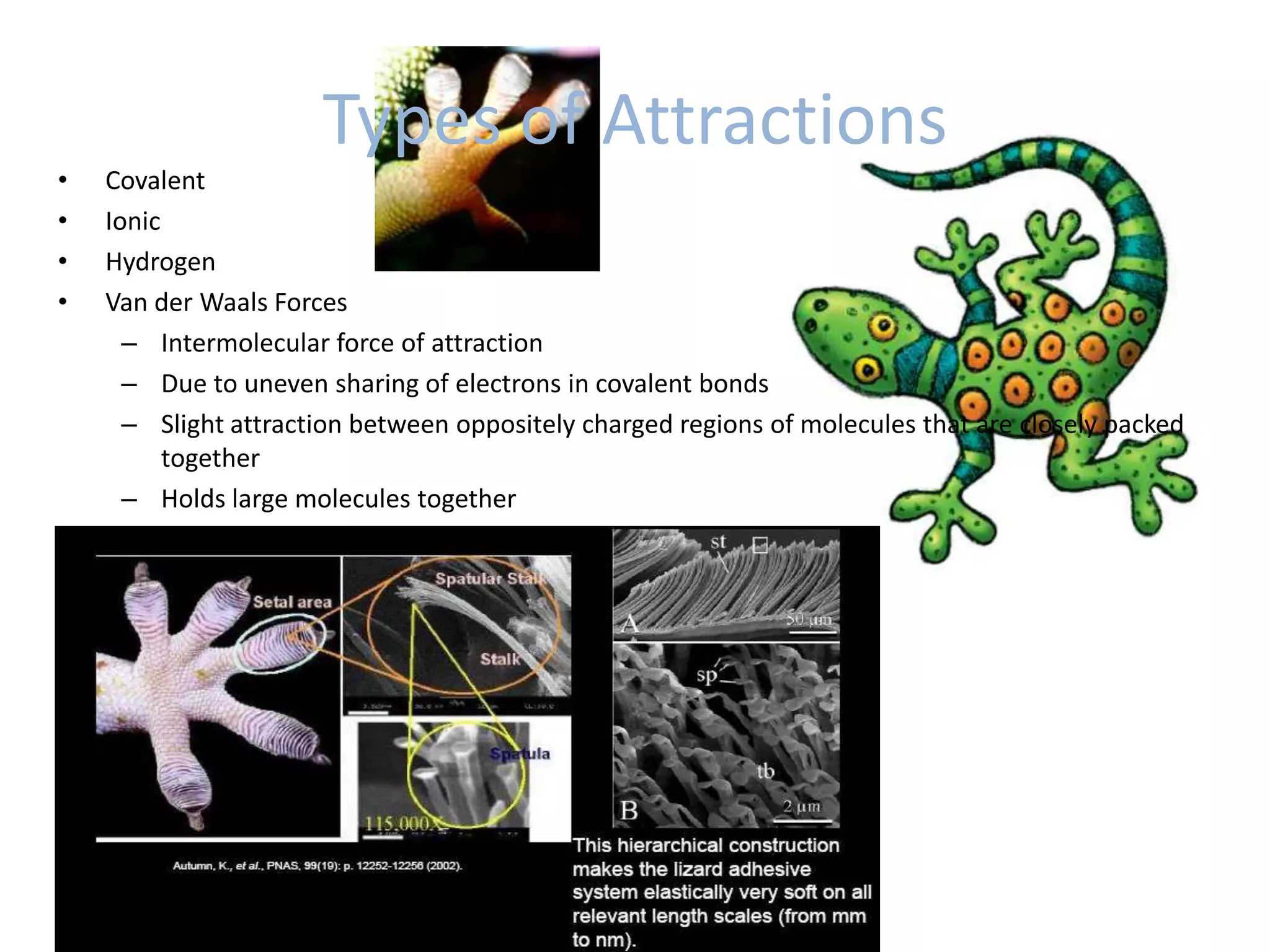
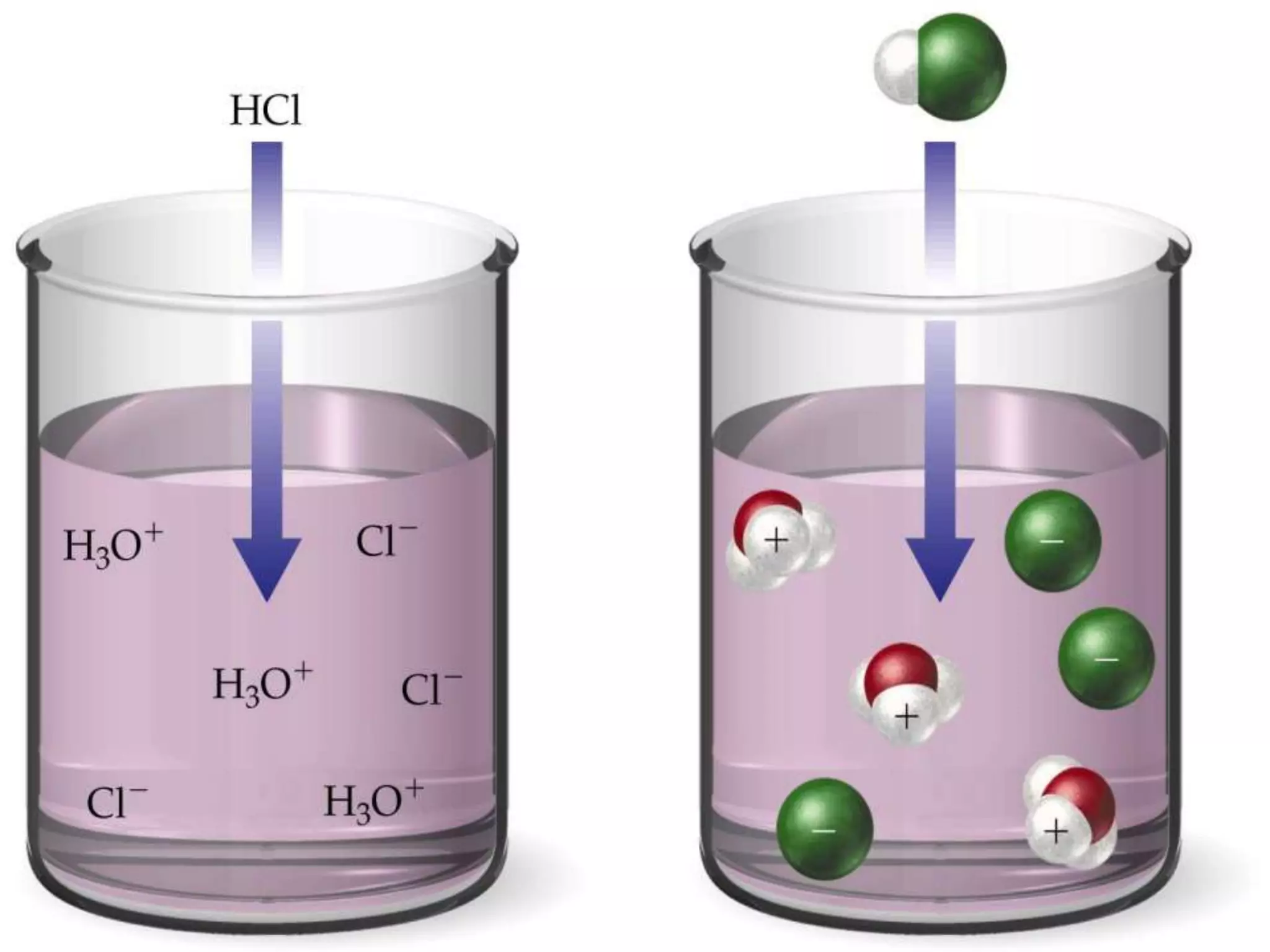
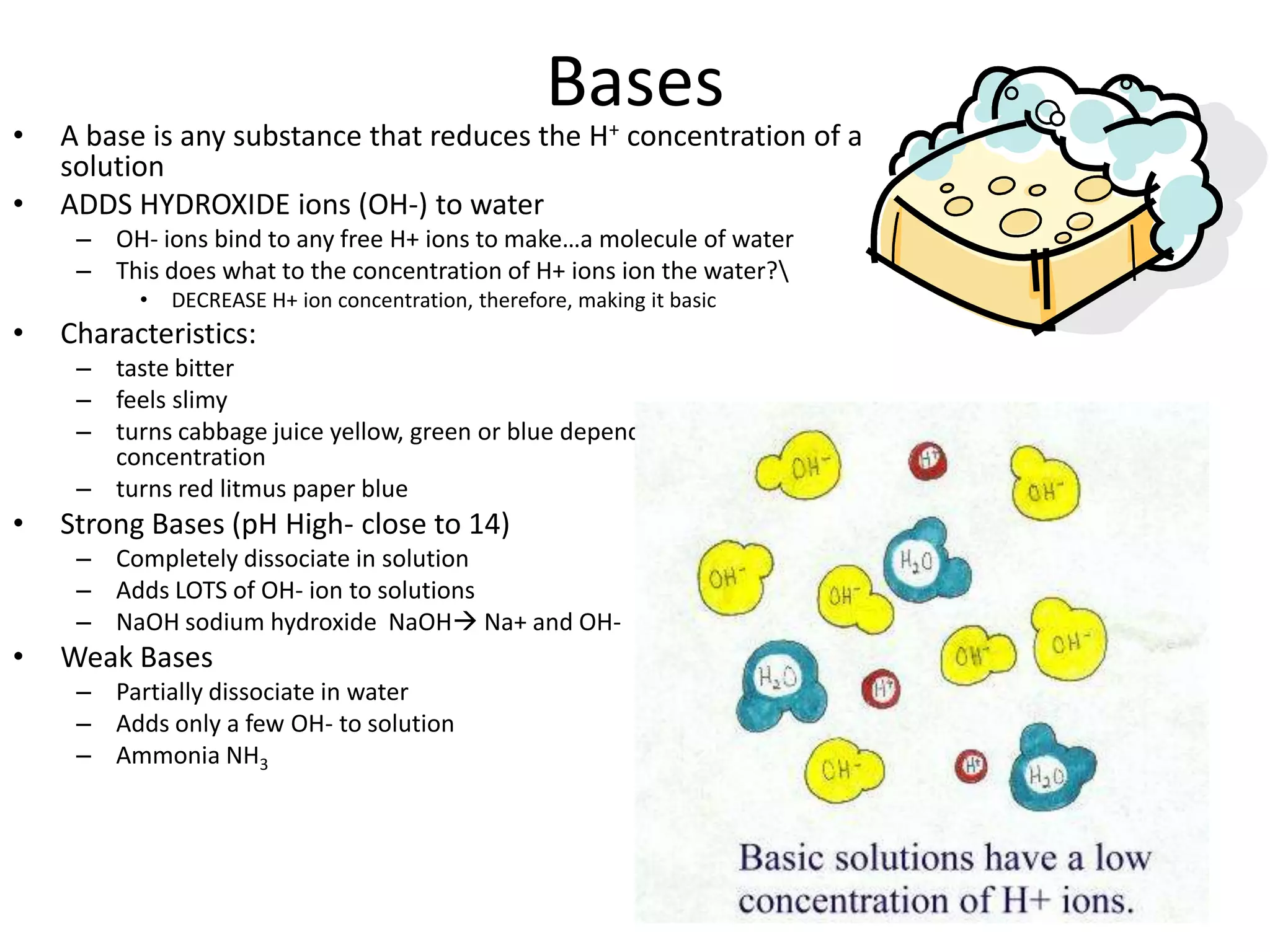
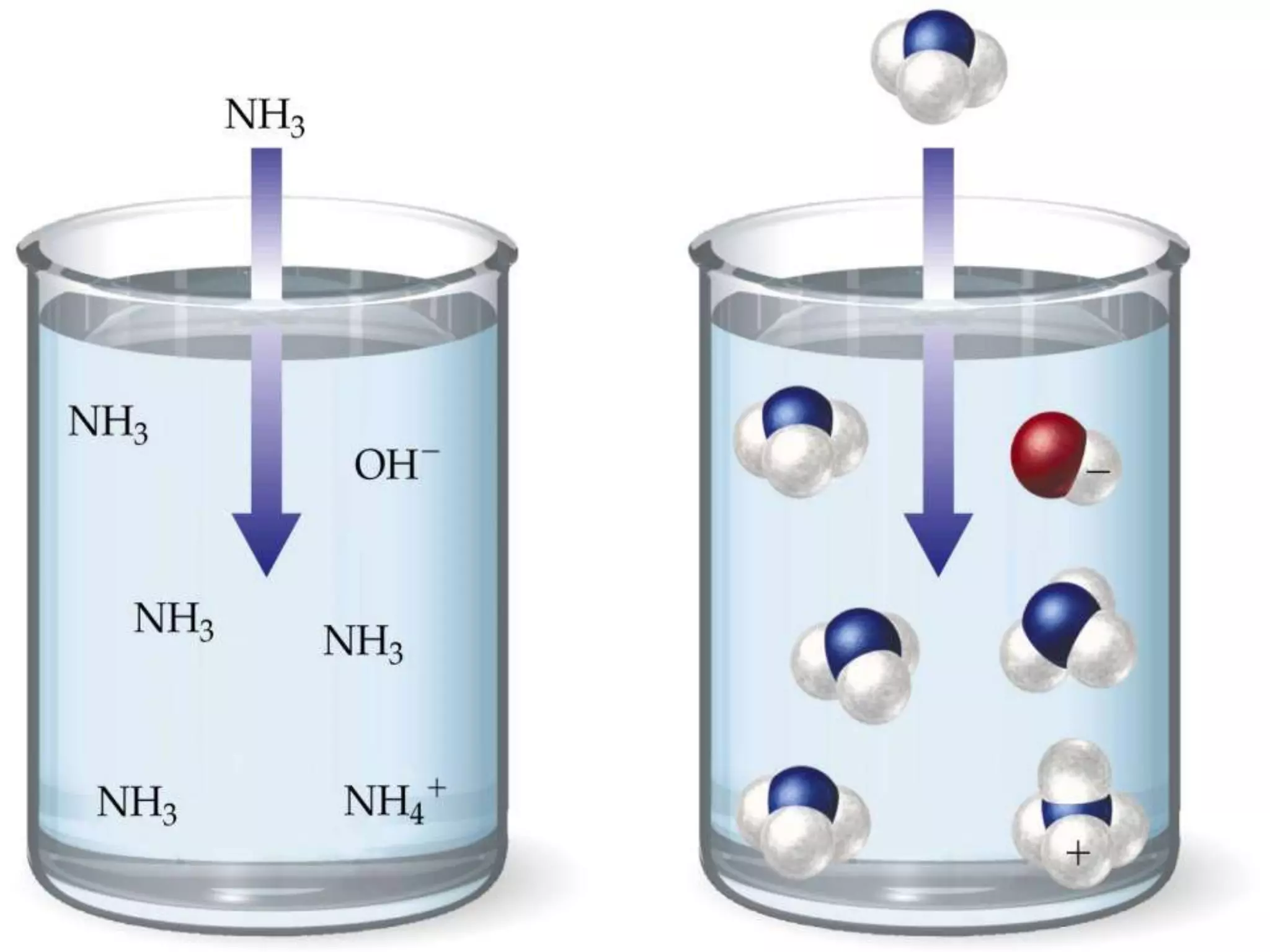
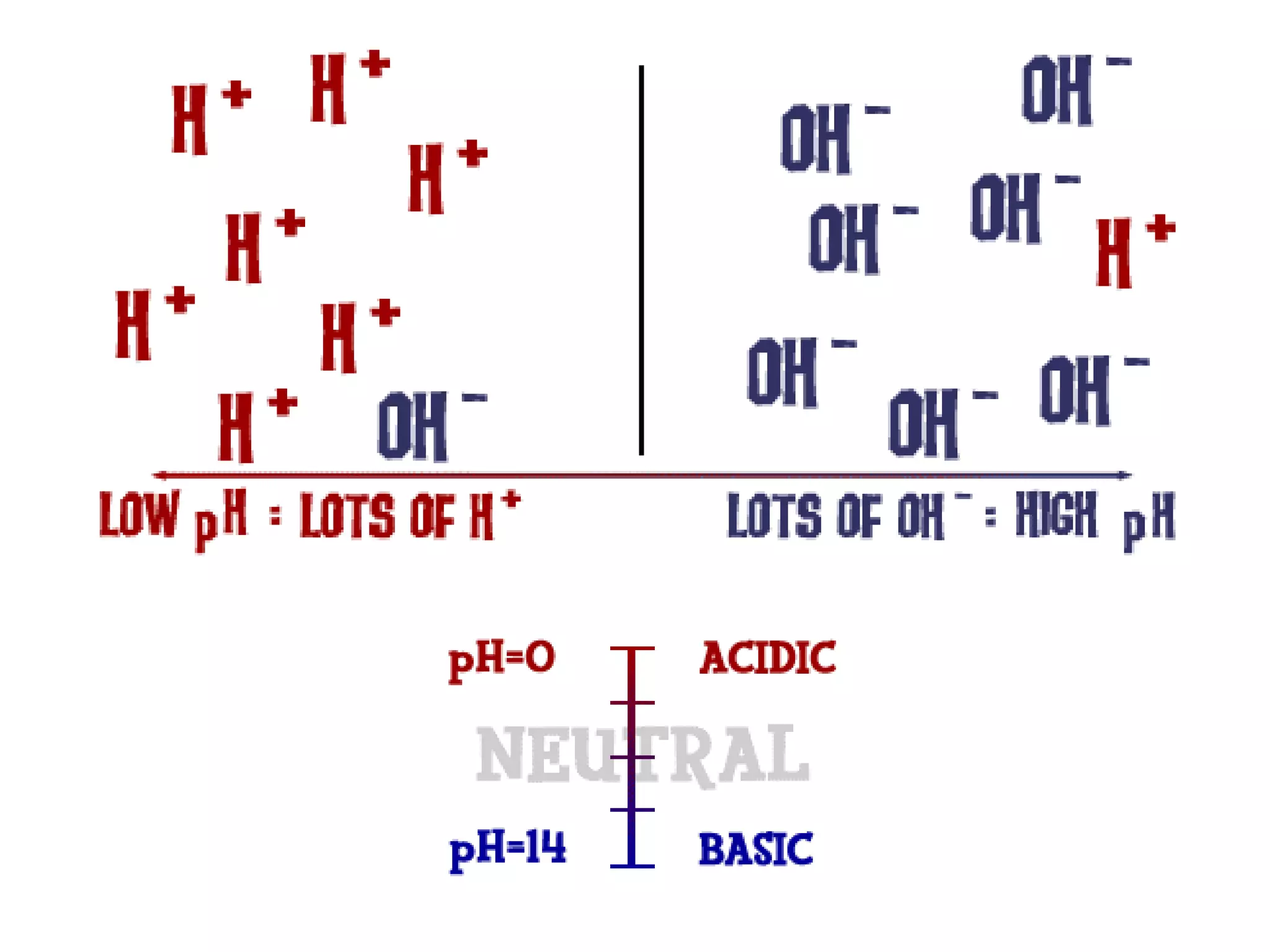
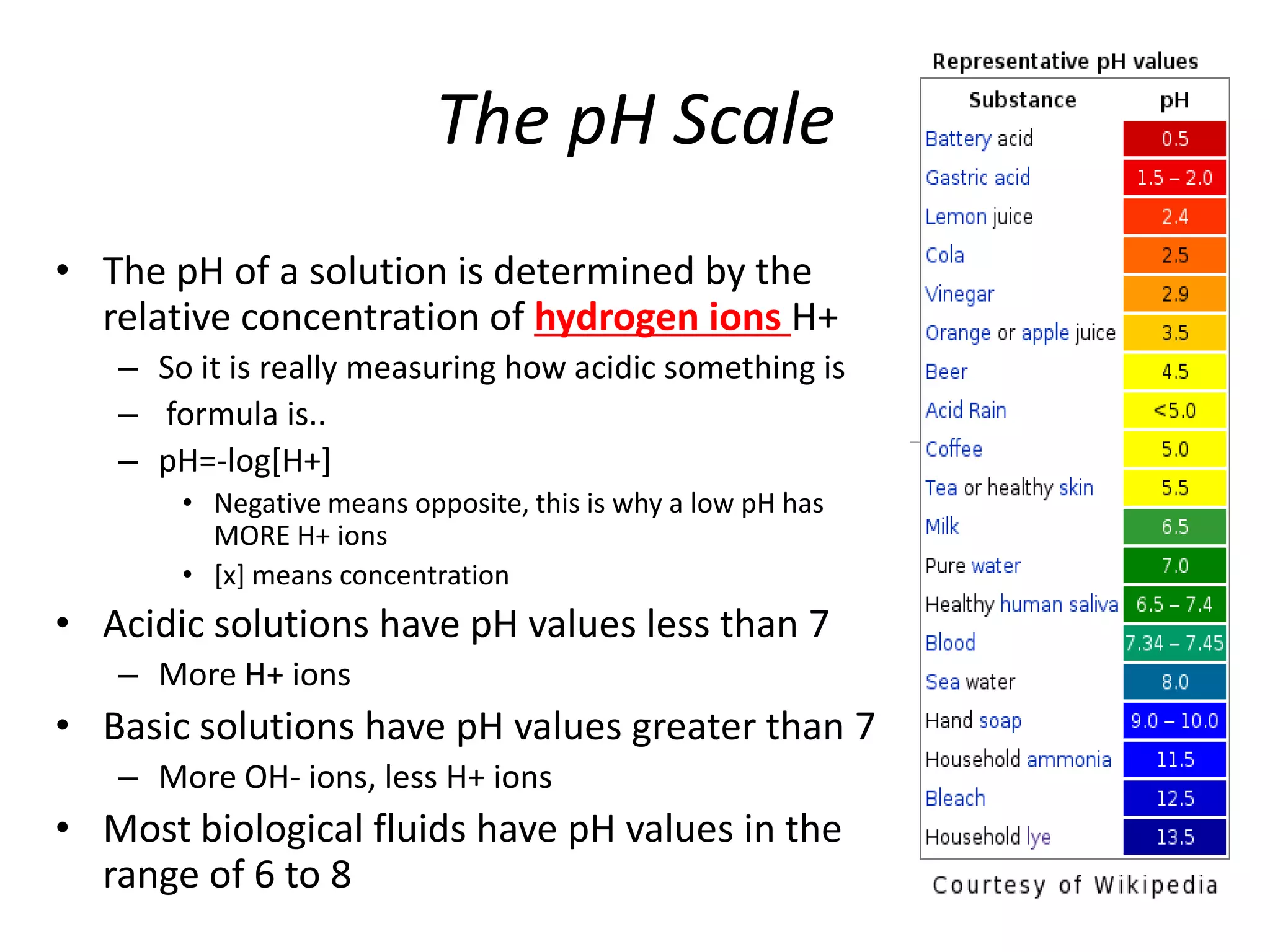
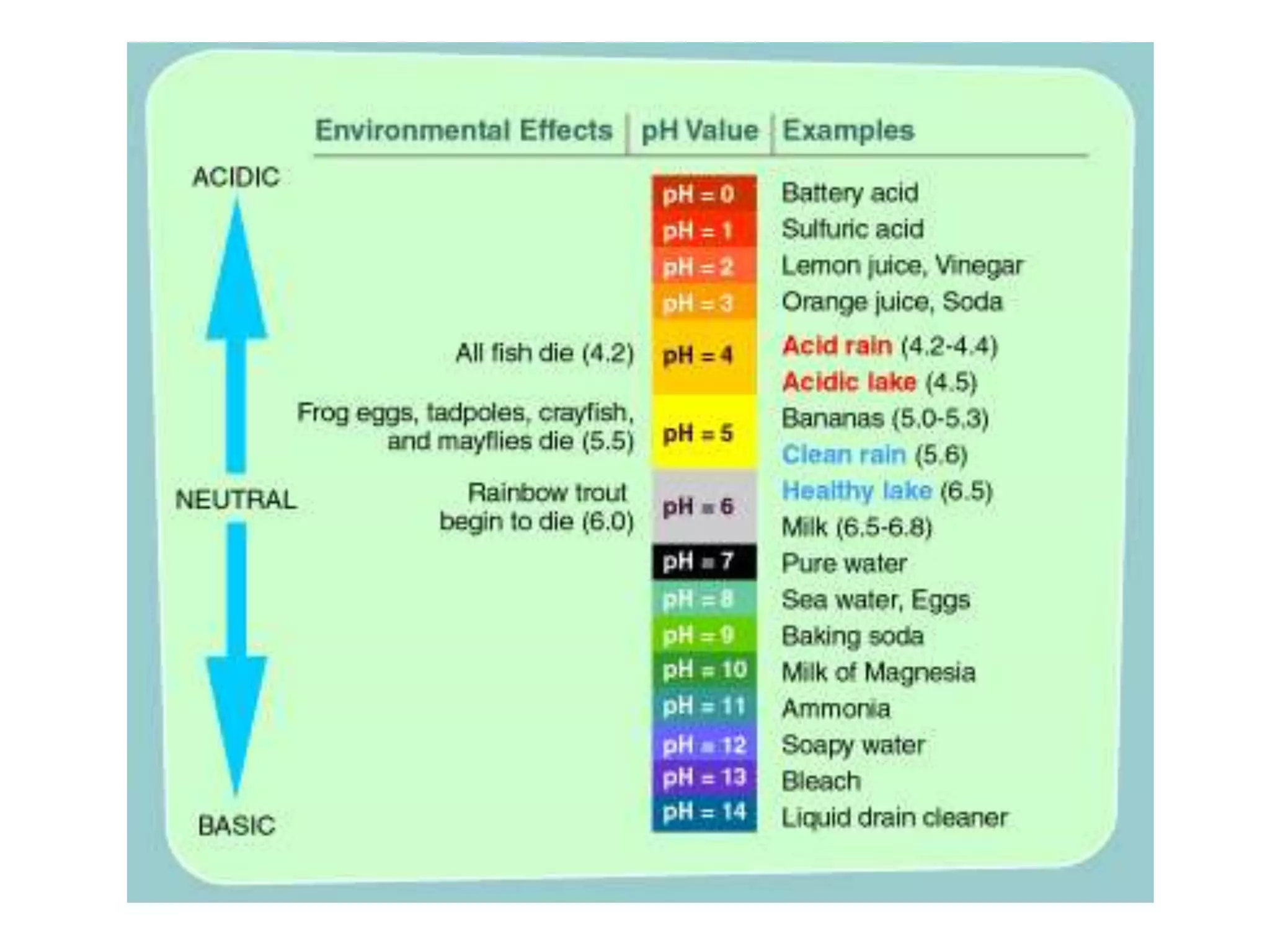
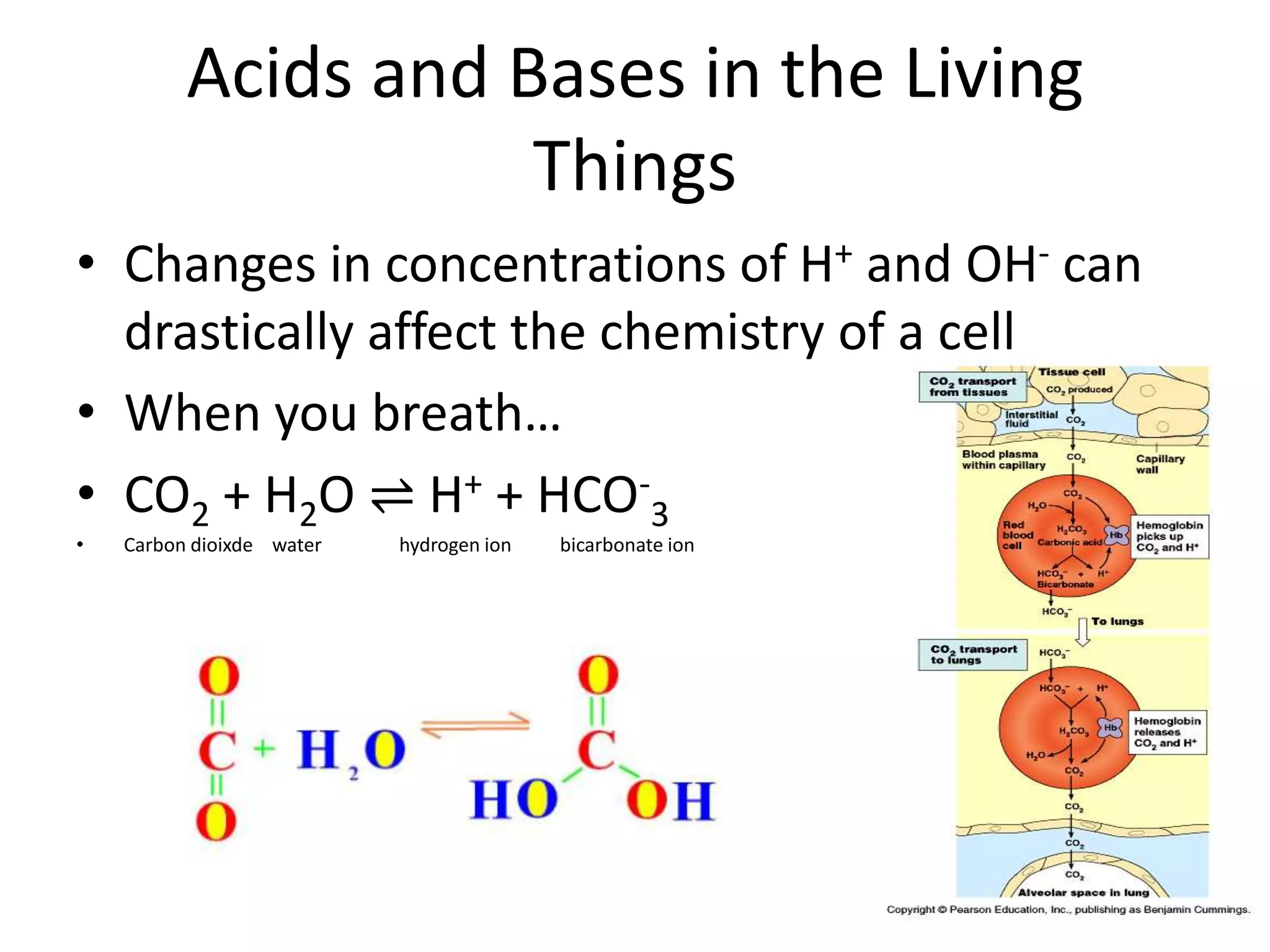
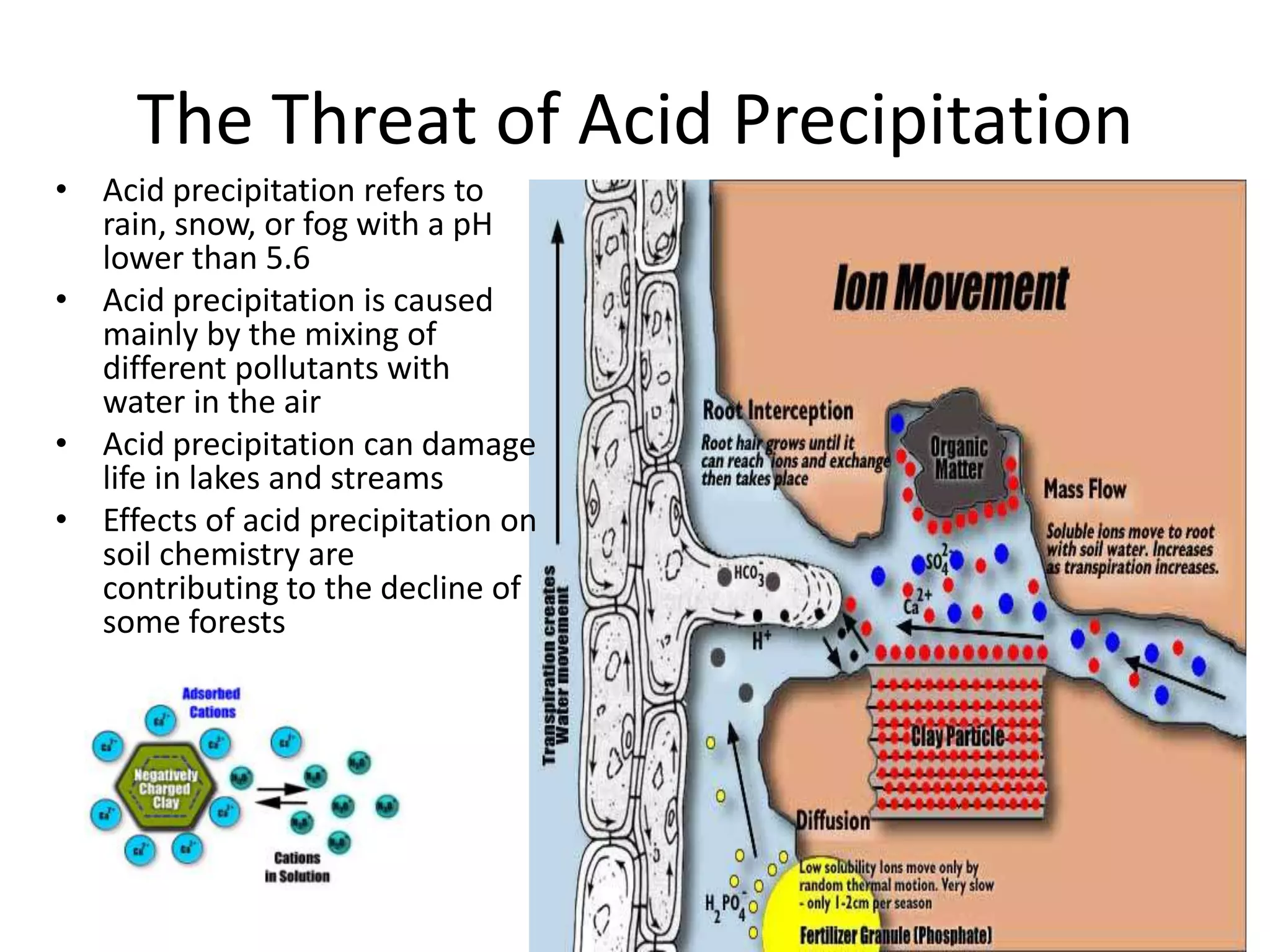
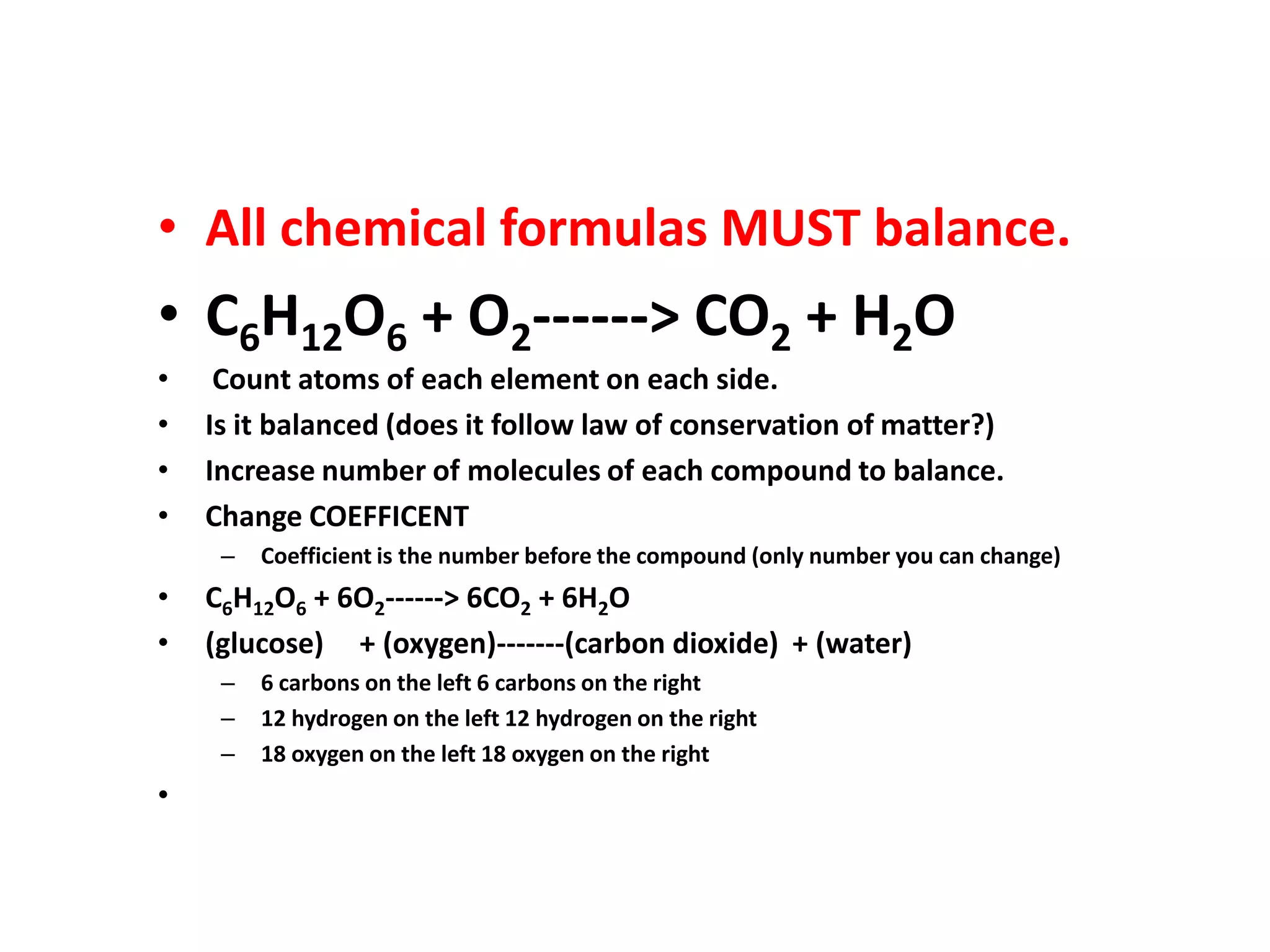
Water is a polar molecule that readily forms hydrogen bonds between its oxygen and hydrogen atoms. This allows water to act as a versatile solvent and absorb large amounts of heat, properties important for life. Water's hydrogen bonding and polarity enable it to dissolve many other polar substances and ions, making it the universal solvent. Its high heat capacity moderates temperatures and allows living organisms to regulate their internal environments. Water's unique physical properties, including its surface tension and ability to absorb heat, are crucial for biological and ecological functions on Earth.