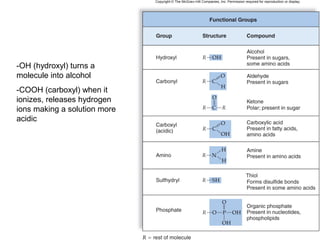
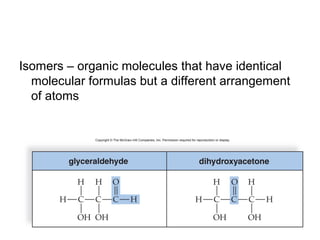
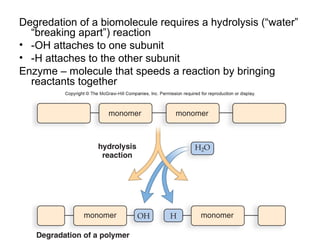
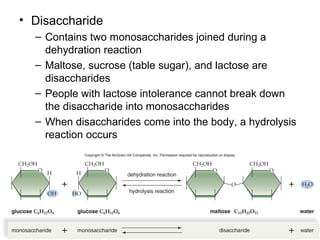
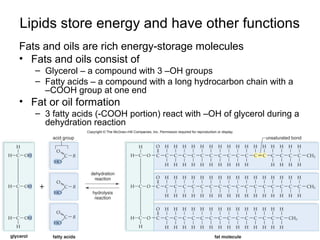
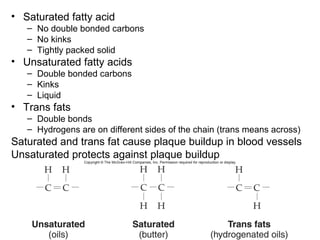
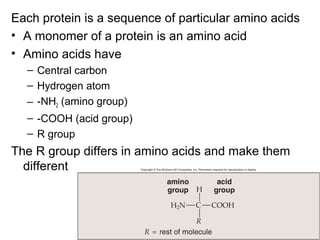
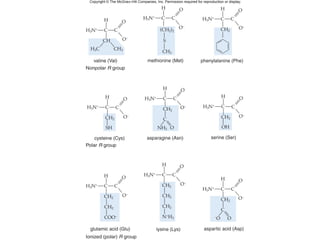
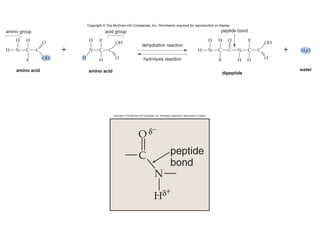
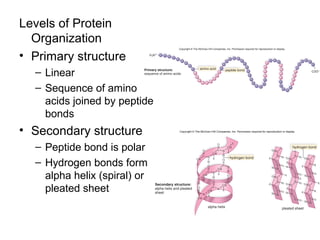
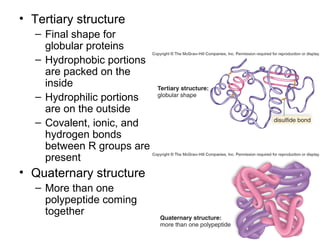
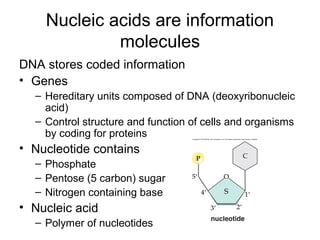
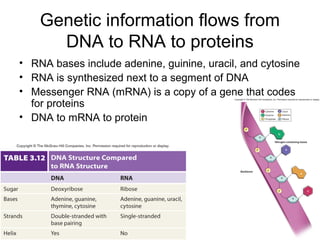
Organic molecules like carbohydrates, lipids, proteins, and nucleic acids are made up of carbon chains and functional groups that allow for great diversity. Carbon forms the backbone of these biomolecules and its ability to form single, double, or triple bonds with other elements allows it to link together into large complex structures. These molecules carry out essential functions in cells like energy storage, structure, metabolism, and information transfer. The specific sequences and structures of proteins and nucleic acids are vital to their roles.