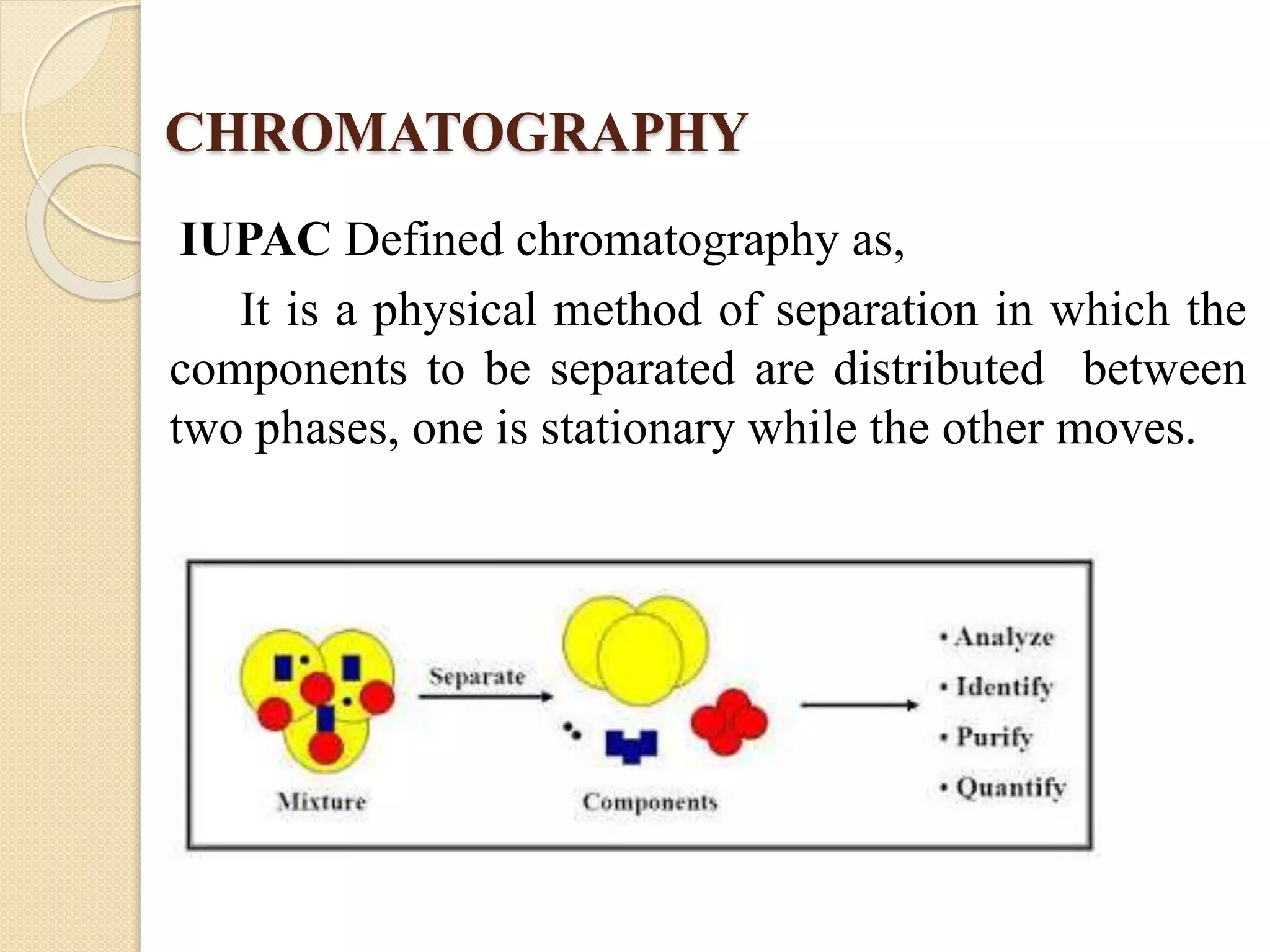
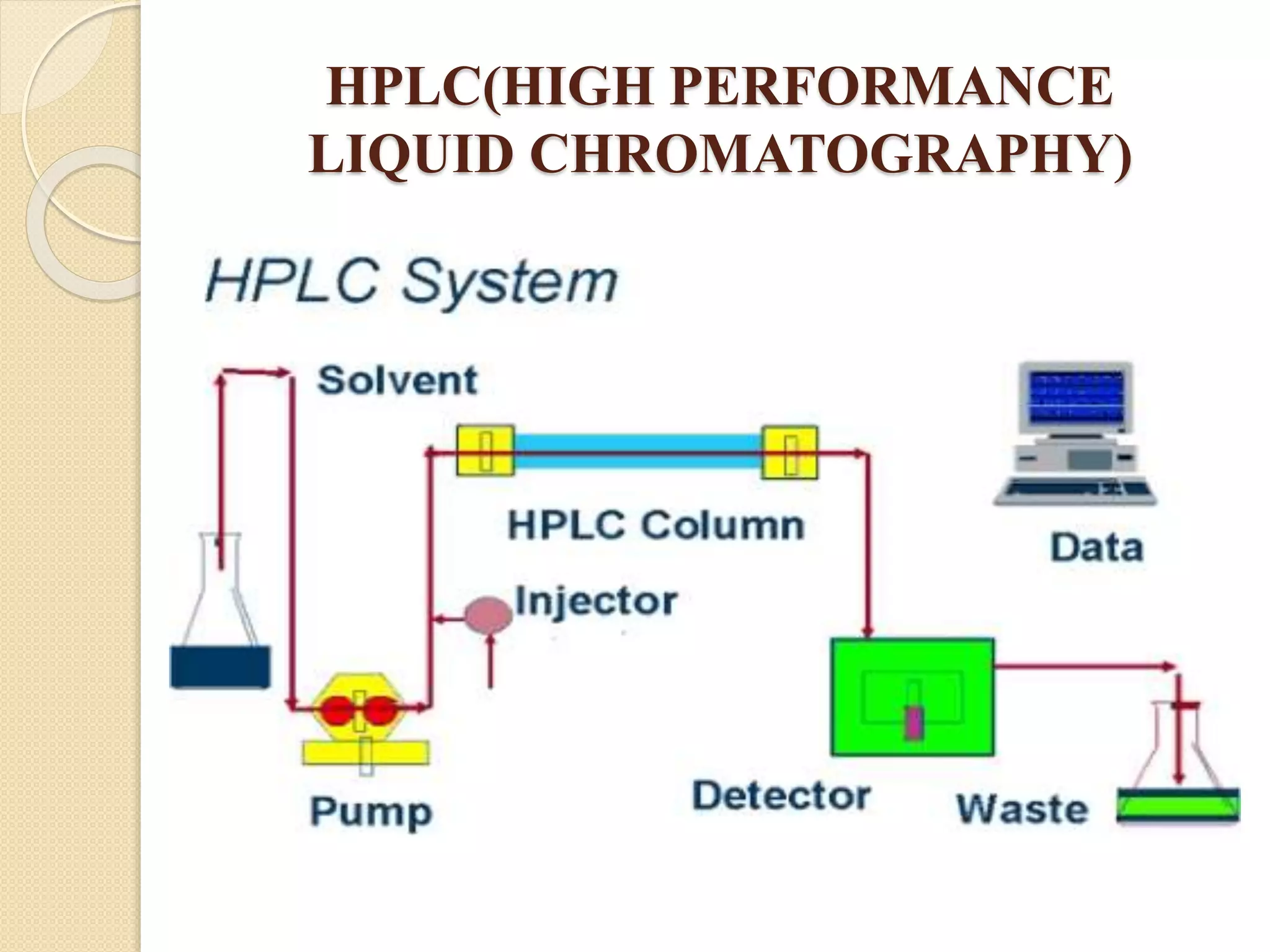
Chromatographic techniques such as thin layer chromatography (TLC), high performance thin layer chromatography (HPTLC), column chromatography, and high performance liquid chromatography (HPLC) separate mixtures by distributing components between a stationary and mobile phase. HPLC uses high pressure to force a mobile liquid or gas phase through a column packed with solid particles. Components elute from the column at different rates and are detected and analyzed. Chromatographic techniques have applications in pharmaceutical analysis, environmental monitoring, food and flavor analysis, and forensics.