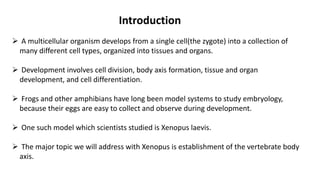
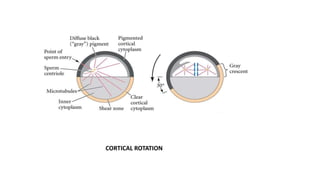
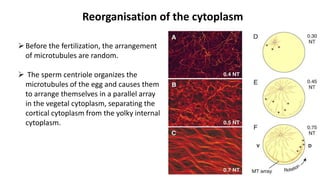
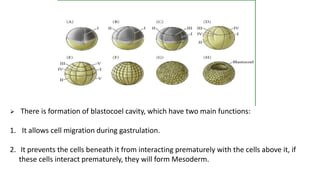
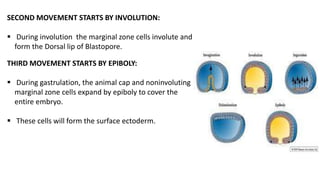
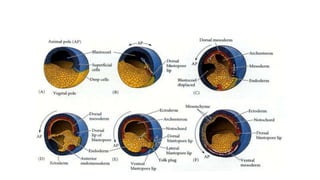
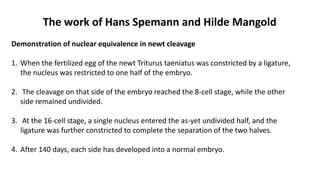
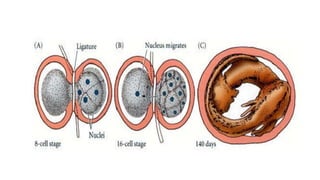
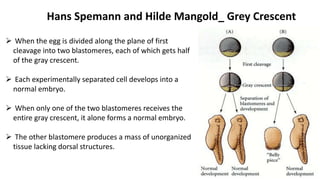
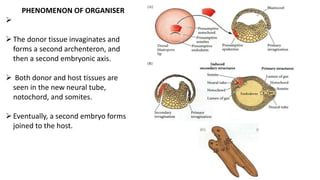
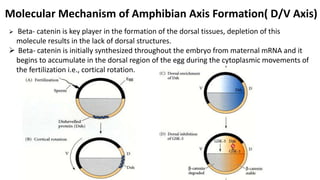
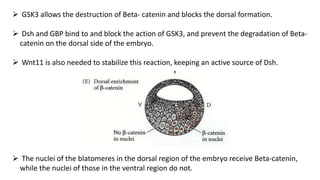
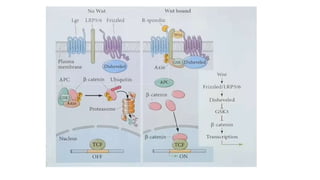
Amphibian development begins with fertilization and cortical rotation that establishes dorsal-ventral polarity. Cleavage is holoblastic but unequal, forming a blastula with large yolky cells in the vegetal pole. Gastrulation involves bottle cell invagination, dorsal mesoderm involution, and ectoderm epiboly. The organizer tissue directs body axis formation through beta-catenin signaling in the dorsal region. Left-right asymmetry results from nodal gene expression induced by cilia rotation in the organizer. Amphibians like Xenopus laevis are widely used models for studying these developmental processes.