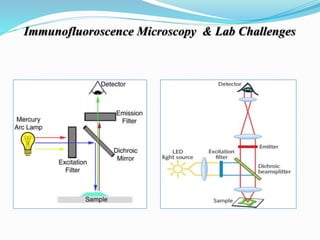
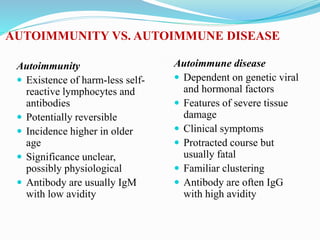
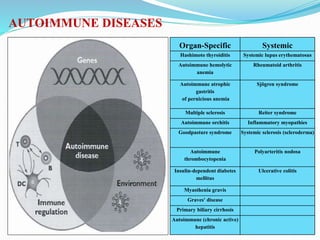
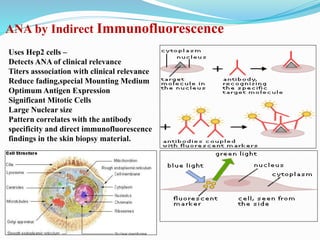
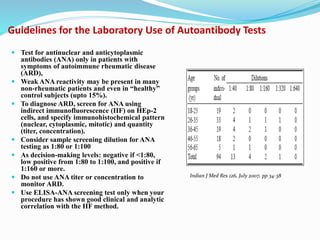
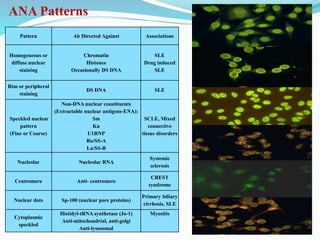
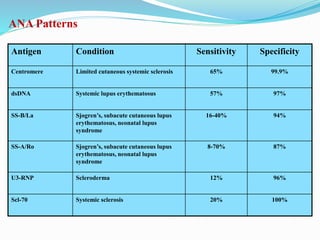
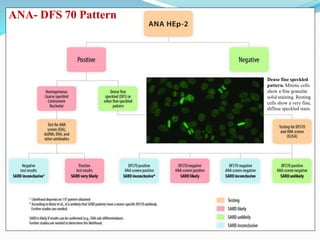
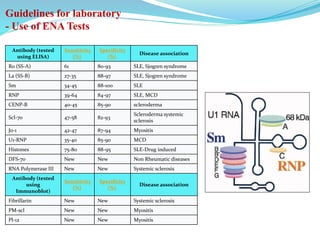
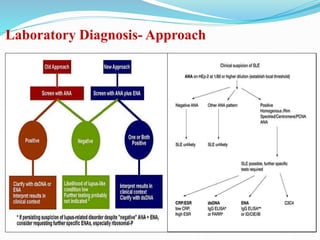
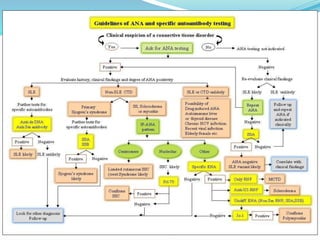
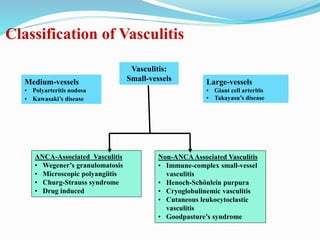
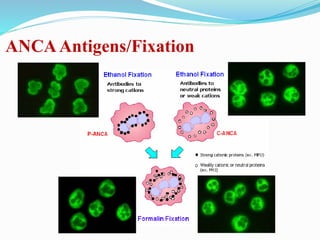
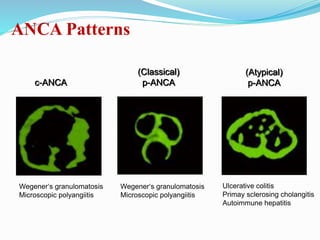
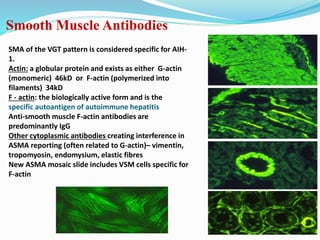
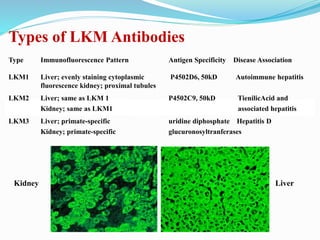
The document discusses the diagnostic challenges associated with autoimmune diseases, highlighting the importance of laboratory tests such as indirect immunofluorescence and specific autoantibody tests. It outlines various autoimmune conditions, their clinical symptoms, and relevant diagnostic methods, while emphasizing the need for careful interpretation of test results in relation to patient symptoms. Guidelines for laboratory use of tests for autoimmune diseases are provided to aid clinicians in making accurate diagnoses.